D,L-Buthionine-(S,R)-sulfoximine
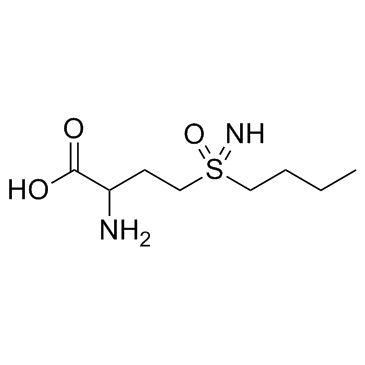
D,L-Buthionine-(S,R)-sulfoximine structure
|
Common Name | D,L-Buthionine-(S,R)-sulfoximine | ||
|---|---|---|---|---|
| CAS Number | 5072-26-4 | Molecular Weight | 222.30500 | |
| Density | 1.29g/cm3 | Boiling Point | 382.3ºC at 760 mmHg | |
| Molecular Formula | C8H18N2O3S | Melting Point | 215ºC (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 185ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of D,L-Buthionine-(S,R)-sulfoximineD,L-Buthionine-(S,R)-sulfoximine is a potent inhibitor of glutamylcysteine synthetase biosynthesis. |
| Name | S-butyl-DL-homocysteine (S,R)-sulfoximine |
|---|---|
| Synonym | More Synonyms |
| Description | D,L-Buthionine-(S,R)-sulfoximine is a potent inhibitor of glutamylcysteine synthetase biosynthesis. |
|---|---|
| Related Catalog | |
| Target |
glutamylcysteine synthetase[1] |
| In Vitro | Buthionine sulfoximine is an analogs of methionine sulfoximine and inhibits gamma-glutamylcysteine synthetase about 20 times more effectively than prothionine sulfoximine and at least 100 times more effectively than methionine sulfoximine[1]. |
| In Vivo | Treatment of mice bearing HT1080 and HT1080/DR4 xenografts with a continuous i.v infusion of nontoxic doses of D,L-Buthionine-(S,R)-sulfoximine (300 and 600 mg/kg/day) produce a 60% reduction of GSH plasma levels and greater than 95 % reduction in GSH tumor levels in both parental and multidrug-resistant tumors[2]. |
| Animal Admin | Mice[2] D,L-Buthionine-(S,R)-sulfoximine is dissolved in sterile 0.9% saline, filtered through a 0.2-p.m polysulfone membrane filter, and administered by 48-h continuous iv. infusion at a dose of 300 mg/kg/day and 600 mg/kg/day starting at 24 h before doxorubicin administration. In vivo GSH levels after treatment with D,L-Buthionine-(S,R)-sulfoximine at a dose of 300 mg/kg and 600 mg/kg for 24 h as an iv. continuous infusion in munine plasma and in tumor tissue of HT1080 and HT1080/DR4 xenografts is measured[2]. |
| References |
| Density | 1.29g/cm3 |
|---|---|
| Boiling Point | 382.3ºC at 760 mmHg |
| Melting Point | 215ºC (dec.)(lit.) |
| Molecular Formula | C8H18N2O3S |
| Molecular Weight | 222.30500 |
| Flash Point | 185ºC |
| Exact Mass | 222.10400 |
| PSA | 112.62000 |
| LogP | 2.30100 |
| Index of Refraction | 1.537 |
| InChIKey | KJQFBVYMGADDTQ-UHFFFAOYSA-N |
| SMILES | CCCCS(=N)(=O)CCC(N)C(=O)O |
| Storage condition | 2-8°C |
| Water Solubility | H2O: 50 mg/mL, clear, colorless |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | EK7713435 |
|
Redox regulation of MMP-3/TIMP-1 ratio in intestinal myofibroblasts: Effect of N-acetylcysteine and curcumin
Exp. Cell Res. 323(1) , 77-86, (2014) Matrix metalloproteinases (MMPs) play a critical role in inflammation and ulcerations in gut of Crohn׳s disease (CD) patients. Intestinal subepithelial myofibroblasts (ISEMFs) secrete MMPs in response... |
|
|
Inhibition of glutathione synthesis distinctly alters mitochondrial and cytosolic redox poise.
Exp. Biol. Med. (Maywood.) 239(4) , 394-403, (2014) The glutathione couple GSH/GSSG is the most abundant cellular redox buffer and is not at equilibrium among intracellular compartments. Perturbation of glutathione poise has been associated with tumori... |
|
|
Isoliquiritigenin attenuates oxidative hepatic damage induced by carbon tetrachloride with or without buthionine sulfoximine.
Chem. Biol. Interact. 225 , 13-20, (2015) Glycyrrhizae radix (G. radix) has been demonstrated to have hepatoprotective properties. This study determined the therapeutic effects of isoliquiritigenin (isoLQ) in G. radix, against liver injury in... |
| 2-amino-4-(butylsulfonimidoyl)butanoic acid |
| DL-Buthionine (S,R)-sulfoximine |
| EINECS 246-685-1 |
| MFCD00070309 |
| D,L-Buthionine-(S,R)-sulfoximine |