Organic Letters
2010-04-02
Copper-catalyzed diacetoxylation of olefins using PhI(OAc)2 as oxidant.
Jayasree Seayad, Abdul Majeed Seayad, Christina L L Chai
Index: Org. Lett. 12(7) , 1412-5, (2010)
Full Text: HTML
Abstract
Copper(I) or -(II) salts with weakly coordinating anions catalyze the diacetoxylation of olefins efficiently in the presence of PhI(OAc)(2) as the oxidant under mild conditions. The reaction is effective for aryl, aryl alkyl, as well as aliphatic terminal and internal olefins forming the corresponding vicinal diacetoxy compounds in 70-85% yields and dr (syn/anti) of up to 5.2. Under these conditions, homoallylic alcohols formed the corresponding tetrahydrofuran derivatives in high yields.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
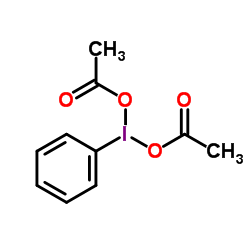 |
(Diacetoxyiodo)benzene
CAS:3240-34-4 |
C10H11IO4 |
Related Articles:
More...
|
An expedient procedure for the oxidative cleavage of olefini...
2010-04-02 [Org. Lett. 12(7) , 1552-5, (2010)] |
|
Palladium-catalyzed oxygenation of unactivated sp3 C-H bonds...
2004-08-11 [J. Am. Chem. Soc. 126 , 9542-9543, (2004)] |
|
Determination of vitamin C in tablets and fruits by titratio...
1982-01-01 [Il Farmaco 37(1) , 9-14, (1982)] |
|
Synthesis of benzimidazoles by PIDA-promoted direct C(sp2)-H...
2012-10-29 [Chemistry 18(44) , 13964-7, (2012)] |
|
Structural studies on bioactive compounds. 32. Oxidation of ...
2000-04-20 [J. Med. Chem. 43(8) , 1550-62, (2000)] |