Chemistry: A European Journal
2012-10-29
Synthesis of benzimidazoles by PIDA-promoted direct C(sp2)-H imidation of N-arylamidines.
Jinbo Huang, Yimiao He, Yong Wang, Qiang Zhu
Index: Chemistry 18(44) , 13964-7, (2012)
Full Text: HTML
Abstract
A metal-free synthesis of diversified benzimidazoles from N-arylamidines through a phenyliodine(III) diacetate (PIDA) promoted intramolecular direct C(sp(2))-H imidation has been developed. The reaction proceeds smoothly at 0 °C or ambient temperature to provide the desired products in good to excellent yields. The synthesis of 2-alkyl- or 2-alkyl-fused benzimidazoles, which are generally inaccessible by similar Pd- or Cu-catalyzed approaches, can also be achieved.Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
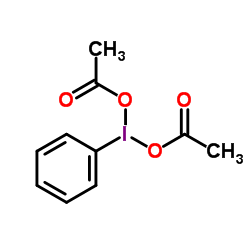 |
(Diacetoxyiodo)benzene
CAS:3240-34-4 |
C10H11IO4 |
Related Articles:
More...
|
An expedient procedure for the oxidative cleavage of olefini...
2010-04-02 [Org. Lett. 12(7) , 1552-5, (2010)] |
|
Palladium-catalyzed oxygenation of unactivated sp3 C-H bonds...
2004-08-11 [J. Am. Chem. Soc. 126 , 9542-9543, (2004)] |
|
Determination of vitamin C in tablets and fruits by titratio...
1982-01-01 [Il Farmaco 37(1) , 9-14, (1982)] |
|
Copper-catalyzed diacetoxylation of olefins using PhI(OAc)2 ...
2010-04-02 [Org. Lett. 12(7) , 1412-5, (2010)] |
|
Structural studies on bioactive compounds. 32. Oxidation of ...
2000-04-20 [J. Med. Chem. 43(8) , 1550-62, (2000)] |