(Diacetoxyiodo)benzene
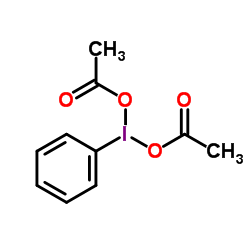
(Diacetoxyiodo)benzene structure
|
Common Name | (Diacetoxyiodo)benzene | ||
|---|---|---|---|---|
| CAS Number | 3240-34-4 | Molecular Weight | 322.096 | |
| Density | 1.6865 | Boiling Point | 456.8ºC at 760 mmHg | |
| Molecular Formula | C10H11IO4 | Melting Point | 161-165 ºC | |
| MSDS | Chinese USA | Flash Point | 230.1ºC | |
|
An expedient procedure for the oxidative cleavage of olefinic bonds with PhI(OAc)2, NMO, and catalytic OsO4.
Org. Lett. 12(7) , 1552-5, (2010) PhI(OAc)(2) in the presence of OsO(4) (cat.) and 2,6-lutidine cleaves olefinic bonds to yield the corresponding carbonyl compounds, albeit, in some cases, with alpha-hydroxy ketones as byproduct. A more practical and clean protocol to effect oxidative cleavag... |
|
|
Palladium-catalyzed oxygenation of unactivated sp3 C-H bonds.
J. Am. Chem. Soc. 126 , 9542-9543, (2004) This communication describes a new palladium-catalyzed method for the oxygenation of unactivated sp3 C-H bonds. A wide variety of alkane substrates containing readily available oxime and/or pyridine directing groups are oxidized with extremely high levels of ... |
|
|
Determination of vitamin C in tablets and fruits by titration with phenyliodosoacetate.
Il Farmaco 37(1) , 9-14, (1982)
|
|
|
Copper-catalyzed diacetoxylation of olefins using PhI(OAc)2 as oxidant.
Org. Lett. 12(7) , 1412-5, (2010) Copper(I) or -(II) salts with weakly coordinating anions catalyze the diacetoxylation of olefins efficiently in the presence of PhI(OAc)(2) as the oxidant under mild conditions. The reaction is effective for aryl, aryl alkyl, as well as aliphatic terminal and... |
|
|
Synthesis of benzimidazoles by PIDA-promoted direct C(sp2)-H imidation of N-arylamidines.
Chemistry 18(44) , 13964-7, (2012) A metal-free synthesis of diversified benzimidazoles from N-arylamidines through a phenyliodine(III) diacetate (PIDA) promoted intramolecular direct C(sp(2))-H imidation has been developed. The reaction proceeds smoothly at 0 °C or ambient temperature to prov... |
|
|
Structural studies on bioactive compounds. 32. Oxidation of tyrphostin protein tyrosine kinase inhibitors with hypervalent iodine reagents.
J. Med. Chem. 43(8) , 1550-62, (2000) Hydroxylated styrenes (tyrphostins) undergo oxidation by hypervalent iodine oxidants such as [(diacetoxy)iodo]benzene (DAIB) to give a range of products depending on the structure of the phenolic substrate, the solvent, the oxidant stoichiometry, and the puri... |
|
|
Substrate dependence of nonlinear effects: mechanistic probe and practical applications.
J. Am. Chem. Soc. 123(22) , 5378-9, (2001)
|
|
|
First synthesis of 3-aryl-4-unsubstituted-6-CF(3)-pyridin-2-ones via aryl migration reaction in the presence of PhI(OAc)(2)/NaOH.
Chem. Commun. (Camb.) 46(37) , 6941-3, (2010) 3-Aryl-4-unsubstituted-6-CF(3)-pyridin-2-ones have been efficiently synthesized from readily available 4-aryl-3-carbamoyl-6-CF(3)-pyridin-2(1H)-ones by treatment with PhI(OAc)(2) in the presence of NaOH. |
|
|
A one-pot oxidative decarboxylation-Friedel-Crafts reaction of acyclic alpha-amino acid derivatives activated by the combination of iodobenzene diacetate/iodine and iron dust.
Org. Biomol. Chem. 6(24) , 4615-21, (2008) An efficient one-pot oxidative decarboxylation-Friedel-Crafts reaction of acyclic alpha-amino acid derivatives with electron-rich aromatic compounds is reported. The reaction is activated by the combination of iodobenzene diacetate, iodine and iron dust, resu... |
|
|
Reactions of morphine derivatives with phenyliodo(III)diacetate (PIDA): synthesis of new morphine analogues.
Curr. Med. Chem. 8(6) , 621-6, (2001) The reactions of morphine and its derivatives with phenyliodo(III)diacetate (PIDA) have been studied. This methodology has not been introduced to morphine alkaloids, despite the fact that such a strategy would ensure dearomatization of the electrophilic aroma... |