2-(N-Fmoc)-3-(N-Boc-N-methoxy)-diaminopropanoic acid, an amino acid for the synthesis of mimics of O-linked glycopeptides.
Michael R Carrasco, Ryan T Brown, Vu H Doan, Sean M Kandel, Franklin C Lee
Index: Biopolymers 84(4) , 414-20, (2006)
Full Text: HTML
Abstract
Amino acids with N-alkylaminooxy side chains have proven effective for the rapid synthesis of neoglycopeptides. Chemoselective reaction of reducing sugars with peptides containing these amino acids provides glycoconjugates that are structurally similar to their natural counterparts. 2-(N-Fmoc)-3-(N-Boc-N-methoxy)-diaminopropanoic acid (Fmoc: 9-fluorenylmethoxycarbonyl; Boc: t-butyloxycarbonyl) was synthesized from Boc-Ser-OH in >40% overall yield and incorporated into peptides by standard Fmoc chemistry based solid phase peptide synthesis. The resulting peptides are efficiently glycosylated and serve as mimics of O-linked glycopeptides. The synthesis of this derivative greatly expands the availability of the N-alkylaminooxy strategy for neoglycopeptides.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
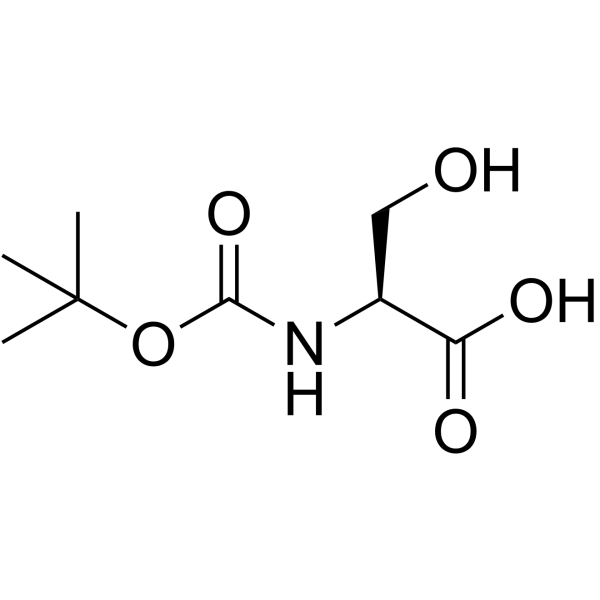 |
Boc-Ser-OH
CAS:3262-72-4 |
C8H15NO5 |
|
Unraveling the interplay of backbone rigidity and electron r...
2014-09-03 [J. Am. Chem. Soc. 136(35) , 12479-88, (2014)] |
|
Transport and signaling via the amino acid binding site of t...
2009-01-01 [Nat. Chem. Biol. 5 , 45-52, (2009)] |
|
In situ fabrication of cleavable peptide arrays on polydimet...
2015-03-20 [Anal. Chim. Acta 865 , 53-9, (2015)] |
|
Design of novel and selective inhibitors of urokinase-type p...
2004-08-06 [J. Biol. Chem. 279(32) , 33613-22, (2004)] |
|
alpha-Amino acid derivatives as proton pump inhibitors and p...
2007-03-01 [Eur. J. Med. Chem. 42 , 386-93, (2007)] |