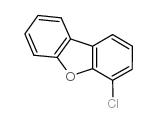
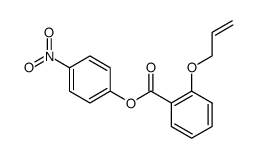
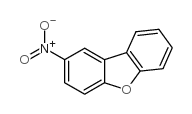
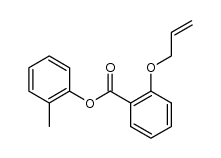
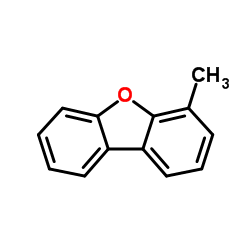
|
~% |
|
~% |
|
~% |
|
~40% |
|
~58% |
|
~% |
|
~% |
|
~72% |
|
~92% |
|
~% |
|
~% |
|
~92% |
|
~30% |
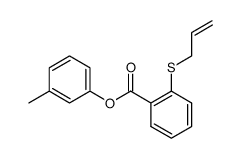
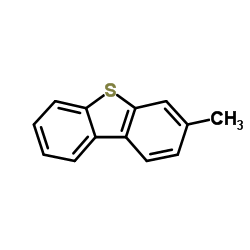
![4-Methyldibenzo[b,d]thiophene Structure](https://image.chemsrc.com/caspic/164/31317-07-4.png)
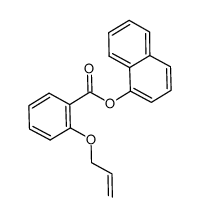
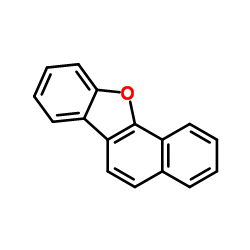
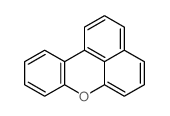
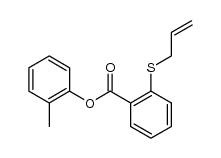
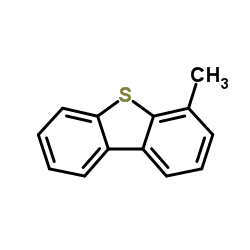


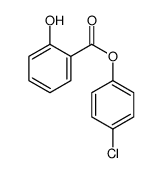

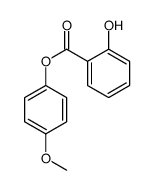
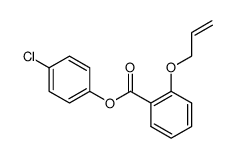
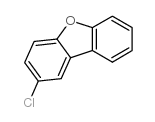

![4-Methyldibenzo[b,d]thiophene Structure](https://image.chemsrc.com/caspic/280/20928-02-3.png)