Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
2004-10-01
A novel method for preparation of cobalt(II) and lead(II) carbonates.
M S Refat, S M Teleb, S A Sadeek
Index: Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 60(12) , 2803-5, (2004)
Full Text: HTML
Abstract
Cobalt(II) carbonate, CoCO3.4H2O and lead(II) carbonate, PbCO3.2H2O were synthesis by a new simple method during the reaction of aqueous solutions of CoX2 (X=Cl-, NO3- and CH3COO-) and PbX2 (X=NO3- or CH3COO-), respectively, with urea at approximately 85 degrees C for 2 h. The infrared spectra of the reaction products clearly indicates the absence of the bands due to coordinated urea, but show the characteristic bands of ionic carbonate. A general mechanisms describing the formation of cobalt and lead carbonates are suggested.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
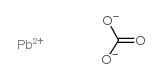 |
LEAD CARBONATE
CAS:598-63-0 |
CO3Pb |
Related Articles:
More...
|
Determination of PbO2 formation kinetics from the chlorinati...
2011-03-15 [Environ. Sci. Technol. 45(6) , 2338-44, (2011)] |
|
Chemical transformations of lead compounds under humid condi...
2013-02-01 [Environ. Geochem. Health 35(1) , 153-9, (2013)] |
|
Comparative effects of various lead salts on delayed hyperse...
1984-10-01 [J. Appl. Toxicol. 4(5) , 265-6, (1984)] |
|
Toxicosis associated with dual oral exposure of rats to lead...
2001-01-01 [Toxicol. Pathol. 29(4) , 451-7, (2001)] |
|
Isotopic characterization of six major brands of white basic...
2002-11-01 [Bull. Environ. Contam. Toxicol. 69(5) , 617-23, (2002)] |