Nickel-NHC-catalyzed alpha-arylation of acyclic ketones and amination of haloarenes and unexpected preferential N-arylation of 4-aminopropiophenone.
Kouki Matsubara, Keita Ueno, Yuji Koga, Kenji Hara
Index: J. Org. Chem. 72 , 5069, (2007)
Full Text: HTML
Abstract
Arylation of both acyclic ketones and primary and secondary amines was achieved using a new, simple, stable, and easy-to-access nickel(II)-halide complex bearing mixed PPh3/N-heterocyclic carbene ligands as a catalyst precursor. Acyclic ketones were first arylated at the alpha-position with the nickel catalyst. On the other hand, less basic amines, such as diphenylamine and 4-aminobenzophenone, were more favorable in the catalytic amination of haloarenes than basic amines, contrary to previous reports. N-Arylation of 4-aminopropiophenone was found to proceed selectively without causing alpha-arylation of the ketone group.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
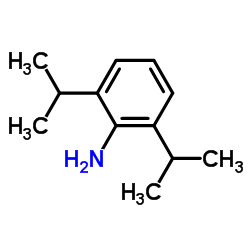 |
2,6-Diisopropylaniline
CAS:24544-04-5 |
C12H19N |
|
Organo-palladium(II) complexes bearing unsymmetrical N,N,N-p...
2015-04-28 [Dalton Trans. 44(16) , 7230-41, (2015)] |
|
O,N,N-pincer ligand effects on oxidatively induced carbon-ch...
2015-04-07 [Dalton Trans. 44(13) , 6040-51, (2015)] |
|
Rigid NON- and NSN-ligand complexes of tetravalent and triva...
2012-07-14 [Dalton Trans. 41(26) , 8175-89, (2012)] |
|
Sulfonated N-heterocyclic carbenes for Suzuki coupling in wa...
2007-07-19 [Chem. Commun. (Camb.) , 2870, (2007)] |
|
Remote activation of nickel catalysts for ethylene oligomeri...
2005-02-04 [Angew. Chem. Int. Ed. Engl. 44(7) , 1108-12, (2005)] |