| Structure | Name/CAS No. | Articles |
|---|---|---|
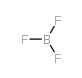 |
bf3
CAS:7637-07-2 |
|
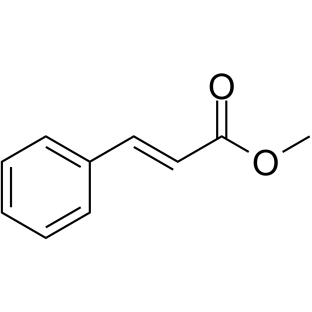 |
Methyl cinnamate
CAS:103-26-4 |
|
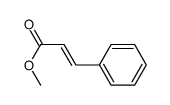 |
methyl (E)-cinnamate
CAS:1754-62-7 |