Methyl cinnamate
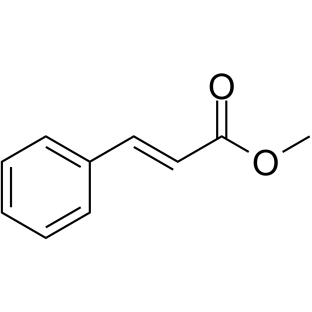
Methyl cinnamate structure
|
Common Name | Methyl cinnamate | ||
|---|---|---|---|---|
| CAS Number | 103-26-4 | Molecular Weight | 162.185 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 261.9±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H10O2 | Melting Point | 34-38 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 141.3±9.9 °C | |
Use of Methyl cinnamateMethyl cinnamate (Methyl 3-phenylpropenoate), an active component of Zanthoxylum armatum, is a widely used natural flavor compound. Methyl cinnamate (Methyl 3-phenylpropenoate) possesses antimicrobial activity and is a tyrosinase inhibitor that can prevent food browning. Methyl cinnamate (Methyl 3-phenylpropenoate) has antiadipogenic activity through mechanisms mediated, in part, by the CaMKK2-AMPK signaling pathway[1]. |
| Name | Methyl cinnamate |
|---|---|
| Synonym | More Synonyms |
| Description | Methyl cinnamate (Methyl 3-phenylpropenoate), an active component of Zanthoxylum armatum, is a widely used natural flavor compound. Methyl cinnamate (Methyl 3-phenylpropenoate) possesses antimicrobial activity and is a tyrosinase inhibitor that can prevent food browning. Methyl cinnamate (Methyl 3-phenylpropenoate) has antiadipogenic activity through mechanisms mediated, in part, by the CaMKK2-AMPK signaling pathway[1]. |
|---|---|
| Related Catalog | |
| In Vitro | In 3T3-L1 cell model, Methyl cinnamate (Methyl 3-phenylpropenoate) inhibits adipocyte differentiation by attenuating expression of the adipogenic transcription factors SREBP-1, PPARγ, and C/EBPα and the transcriptional activity of PPARγ. In addition, Methyl cinnamate (Methyl 3-phenylpropenoate) activates the CaMKK2−AMPK signaling cascade involved in the regulation of adipogenesis[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 261.9±0.0 °C at 760 mmHg |
| Melting Point | 34-38 °C(lit.) |
| Molecular Formula | C10H10O2 |
| Molecular Weight | 162.185 |
| Flash Point | 141.3±9.9 °C |
| Exact Mass | 162.068085 |
| PSA | 26.30000 |
| LogP | 2.18 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.559 |
| InChIKey | CCRCUPLGCSFEDV-BQYQJAHWSA-N |
| SMILES | COC(=O)C=Cc1ccccc1 |
| Storage condition | 0-10°C |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | GE0190000 |
| HS Code | 2916399090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2916399090 |
|---|---|
| Summary | 2916399090 other aromatic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Contribution of cinnamic acid analogues in rosmarinic acid to inhibition of snake venom induced hemorrhage.
Bioorg. Med. Chem. 19 , 2392-6, (2011) In our previous paper, we reported that rosmarinic acid (1) of Argusia argentea could neutralize snake venom induced hemorrhagic action. Rosmarinic acid (1) consists of two phenylpropanoids: caffeic a... |
|
|
BF3·OEt2-promoted diastereoselective diacetoxylation of alkenes by PhI(OAc)2.
J. Org. Chem. 76(24) , 9997-10004, (2011) Selective syn and anti diacetoxylations of alkenes have been achieved using a PhI(OAc)(2)/BF(3)·OEt(2) system in the presence and absence of water, respectively. A broad range of substrates including ... |
|
|
Stoichiometric model of alpha-cyclodextrin complex formation.
J. Pharm. Sci. 69(5) , 564-7, (1980) The solubility, spectral, and kinetic methods were used to study complexing between alpha-cyclodextrin (ligand, L) and 3,5-dimethoxycinnamic acid, benzalacetone, and methyl cinnamate (substrates, S). ... |
| METHYLCINNAMATE |
| METHYL CINANMATE |
| Cinnamic acid methylester |
| E-3-phenyl-acrylic acid methyl ester |
| (E)-cinnamic acid methyl ester |
| TRANS-CINNAMIC ACID METHYL ESTER |
| cinnamic acid methyl ester |
| (2E)-3-Phenyl-2-propenoic acid methyl ester |
| trans-3-Phenylacrylic Acid Methyl Ester |
| METHYL-3-PHENYLPROPENOTE |
| Methyl (2E)-3-phenylacrylate |
| Methyl cinnamate |
| METHYL CINNAMATE/CINNAMIC ACID METHYL ESTER |
| EINECS 203-093-8 |
| Methyl (E)-cinnamate |
| 2-Propenoic acid, 3-phenyl-, methyl ester, (2E)- |
| CINNAMICACIDMETHYLESTER |
| MFCD00008458 |
| Methyl trans-3-Phenylacrylate |
| METHYL 3-PHENYLACRYLATE |
| methyl (2E)-3-phenylprop-2-enoate |
| METHYL CIMNAMATE NATURAL |
| METHYL CINNAMYLATE |
| Methyl trans-cinnamate |
| FEMA 2698 |
 CAS#:21770-48-9
CAS#:21770-48-9 CAS#:67-56-1
CAS#:67-56-1 CAS#:140-10-3
CAS#:140-10-3 CAS#:292638-85-8
CAS#:292638-85-8 CAS#:98-80-6
CAS#:98-80-6 CAS#:71-43-2
CAS#:71-43-2 CAS#:77-78-1
CAS#:77-78-1 CAS#:100-52-7
CAS#:100-52-7 CAS#:96-34-4
CAS#:96-34-4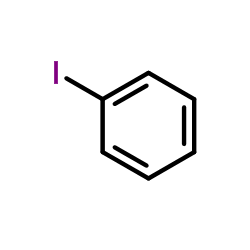 CAS#:591-50-4
CAS#:591-50-4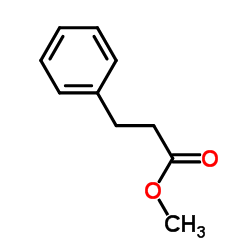 CAS#:103-25-3
CAS#:103-25-3![Bicyclo[8.2.0]dodecan-10-ol-8-on structure](https://image.chemsrc.com/caspic/305/61883-07-6.png) CAS#:61883-07-6
CAS#:61883-07-6 CAS#:10498-83-6
CAS#:10498-83-6 CAS#:37088-66-7
CAS#:37088-66-7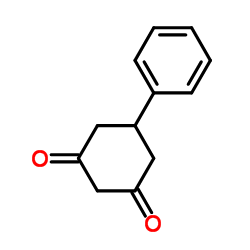 CAS#:493-72-1
CAS#:493-72-1 CAS#:108-94-1
CAS#:108-94-1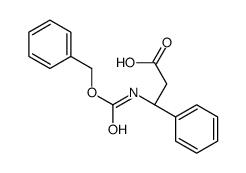 CAS#:14441-08-8
CAS#:14441-08-8 CAS#:939-90-2
CAS#:939-90-2 CAS#:40546-94-9
CAS#:40546-94-9 CAS#:4165-96-2
CAS#:4165-96-2
