| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
2-Pyrrolidinone
CAS:616-45-5 |
|
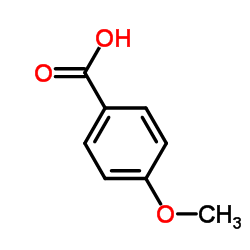 |
p-Anisic acid
CAS:100-09-4 |
|
 |
Aniracetam
CAS:72432-10-1 |