2-Pyrrolidinone

2-Pyrrolidinone structure
|
Common Name | 2-Pyrrolidinone | ||
|---|---|---|---|---|
| CAS Number | 616-45-5 | Molecular Weight | 85.104 | |
| Density | 1.103 | Boiling Point | 250 ºC | |
| Molecular Formula | C4H7NO | Melting Point | 25.5 ºC | |
| MSDS | Chinese USA | Flash Point | 138 ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2-Pyrrolidinone2-Pyrrolidinone is an active compound present in Brassica oleracea var. capitata and displays anticancer property. |
| Name | pyrrolidin-2-one |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Pyrrolidinone is an active compound present in Brassica oleracea var. capitata and displays anticancer property. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Radical scavenging activity increases with increasing the amount of 2-Pyrrolidinone. The cytotoxicity of 2-Pyrrolidinone is found to be dose and time dependent, and the effect is observed microscopically in all of the selected cancer cell lines. It is observed that 2-Pyrrolidinone has cytotoxic concentrations of 2.5 mg/mL for HeLa, 3 mg/mL for PC-3 cells at 24 h and 1.5 mg/mL for HeLa and 2 mg/mL for PC-3 cells at 48 h, respectively. The cell viability decreases with increasing concentrations of purified 2-Pyrrolidinone. After treatment with 2-Pyrrolidinone their IC50 concentrations (1.5 mg/mL and 2 mg/mL for HeLa and PC-3 cells, respectively) for a period of 24 h, morphological changes in the cells are observed. It is demonstrated that 2-Pyrrolidinone induces apoptosis in HeLa and PC-3 cells via G0/G1phase of cell cycle arrest[1]. |
| Cell Assay | The inhibitory concentrations (IC50) are evaluated using an MTT assay. Cancer cells are grown (1×104 cells/well) in a 96-well plate for 48 h into 75% confluence. The medium is replaced with fresh medium containing concentration of (0.5 to 5 mg/mL) 2-Pyrrolidinone, and the cells are further incubated for 24 h and 48 h. The culture medium is removed, and 100 mL of the MTT solution is added to each well and incubated at 37°C for 4 h. After removal of the supernatant, 50 mL of DMSO is added to each of the wells and incubated for 10 min to solubilize the formazan crystals. The optical density is measured at 620 nm in an ELISA multi-well plate reader. The OD value is used to calculate the percentage of viability[1]. |
| References |
| Density | 1.103 |
|---|---|
| Boiling Point | 250 ºC |
| Melting Point | 25.5 ºC |
| Molecular Formula | C4H7NO |
| Molecular Weight | 85.104 |
| Flash Point | 138 ºC |
| Exact Mass | 85.052765 |
| PSA | 29.10000 |
| LogP | -1.01 |
| Vapour density | 2.9 (vs air) |
| Vapour Pressure | 0.1±0.8 mmHg at 25°C |
| Index of Refraction | 1.486-1.488 |
| Storage condition | 2-8°C |
| Water Solubility | miscible |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| Safety Phrases | S24/25 |
| RIDADR | 2810 |
| WGK Germany | 1 |
| RTECS | UY5715000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933790090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933790090 |
|---|---|
| Summary | 2933790090. other lactams. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:9.0%. General tariff:20.0% |
|
Identification of photosynthesis inhibitors of pelagic marine algae using 96-well plate microfractionation for enhanced throughput in effect-directed analysis.
Environ. Sci. Technol. 48(14) , 8003-11, (2014) Because of large-scale production and use of an increasing diversity of chemicals in modern society, estuarine and coastal waters may be contaminated with numerous substances. Some of these compounds ... |
|
|
Gellan gum-g-N-vinyl-2-pyrrolidone: synthesis, swelling, metal ion uptake and flocculation behavior.
Int. J. Biol. Macromol. 72 , 1292-300, (2014) The synthesis of graft copolymer (gellan gum-g-N-vinyl-2-pyrrolidone) is carried out in nitrogen atmosphere using potassium bromate and silver as redox system. The reaction conditions for maximum graf... |
|
|
Formulation and characterization of polymeric films containing combinations of antiretrovirals (ARVs) for HIV prevention.
Pharm. Res. 32(2) , 458-68, (2015) To develop polymeric films containing dual combinations of anti-HIV drug candidate tenofovir, maraviroc and dapivirine for vaginal application as topical microbicides.A solvent casting method was used... |
| 2-Pyrrolidinone |
| 1-azacyclopentan-2-one |
| azacyclopentan-2-one |
| pyrrolidin-2-one |
| pyrrolidinone |
| 2-oxopyrrolidine |
| α-pyrrolidone |
| MFCD00005270 |
| Pyrrolidin-2-on |
| pyrrolidone |
| Tetrahydropyrrolone |
| 2-Pyrrolidone |
| EINECS 210-483-1 |
| Piracetam Impurity 1 |
 CAS#:541-59-3
CAS#:541-59-3 CAS#:2226-88-2
CAS#:2226-88-2 CAS#:123-56-8
CAS#:123-56-8 CAS#:2399-66-8
CAS#:2399-66-8 CAS#:13325-10-5
CAS#:13325-10-5 CAS#:5959-36-4
CAS#:5959-36-4 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:107-11-9
CAS#:107-11-9 CAS#:14468-80-5
CAS#:14468-80-5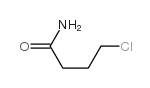 CAS#:2455-04-1
CAS#:2455-04-1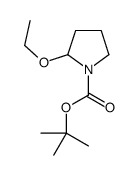 CAS#:106412-40-2
CAS#:106412-40-2![1-[(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]pyrrolidin-2-one structure](https://image.chemsrc.com/caspic/446/110261-31-9.png) CAS#:110261-31-9
CAS#:110261-31-9![Butanoic acid,4-[(2,4-dinitrophenyl)amino]- structure](https://image.chemsrc.com/caspic/253/10466-75-8.png) CAS#:10466-75-8
CAS#:10466-75-8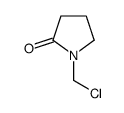 CAS#:31282-95-8
CAS#:31282-95-8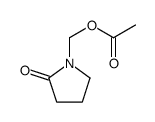 CAS#:109071-38-7
CAS#:109071-38-7 CAS#:3772-26-7
CAS#:3772-26-7 CAS#:3470-99-3
CAS#:3470-99-3 CAS#:3470-98-2
CAS#:3470-98-2 CAS#:143023-95-4
CAS#:143023-95-4