Aniracetam

Aniracetam structure
|
Common Name | Aniracetam | ||
|---|---|---|---|---|
| CAS Number | 72432-10-1 | Molecular Weight | 219.236 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 399.7±34.0 °C at 760 mmHg | |
| Molecular Formula | C12H13NO3 | Melting Point | −58 °C(lit.) | |
| MSDS | Chinese | Flash Point | 195.5±25.7 °C | |
Use of AniracetamAniracetam(Ro 13-5057) is a nootropics and neuroprotective drug, which is selectively modulates the AMPA receptor and nAChR.Target: AMPA; nAChRAniracetam is an ampakine and nootropic of the racetam chemical class purported to be considerably more potent than piracetam. It selectively modulates the AMPA receptor. It is lipid soluble and has possible cognition enhancing effects. It has been tested in animals extensively, Alzheimer's patients and temporarily-impaired healthy subjects. It has shown potential as an anxiolytic in three clinical animal models [1].Administration of aniracetam for 10 days (post-natal days (PND) 18-27), at a dose of 50 mg/kg reversed cognitive deficits in both rat genders, indicated by a significant increase in the number of avoidances and the number of 'good learners'. After the termination of the nootropic treatment, a significant increase in both amplitude and frequency of AMPA receptor-mediated mEPSCs in hippocampal CA-1 pyramidal cells was observed [2].Clinical indications: Cognitive disorder; StrokeFDA Approved Date: Toxicity: insomnia; headaches; nausea or rash. |
| Name | aniracetam |
|---|---|
| Synonym | More Synonyms |
| Description | Aniracetam(Ro 13-5057) is a nootropics and neuroprotective drug, which is selectively modulates the AMPA receptor and nAChR.Target: AMPA; nAChRAniracetam is an ampakine and nootropic of the racetam chemical class purported to be considerably more potent than piracetam. It selectively modulates the AMPA receptor. It is lipid soluble and has possible cognition enhancing effects. It has been tested in animals extensively, Alzheimer's patients and temporarily-impaired healthy subjects. It has shown potential as an anxiolytic in three clinical animal models [1].Administration of aniracetam for 10 days (post-natal days (PND) 18-27), at a dose of 50 mg/kg reversed cognitive deficits in both rat genders, indicated by a significant increase in the number of avoidances and the number of 'good learners'. After the termination of the nootropic treatment, a significant increase in both amplitude and frequency of AMPA receptor-mediated mEPSCs in hippocampal CA-1 pyramidal cells was observed [2].Clinical indications: Cognitive disorder; StrokeFDA Approved Date: Toxicity: insomnia; headaches; nausea or rash. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 399.7±34.0 °C at 760 mmHg |
| Melting Point | −58 °C(lit.) |
| Molecular Formula | C12H13NO3 |
| Molecular Weight | 219.236 |
| Flash Point | 195.5±25.7 °C |
| Exact Mass | 219.089539 |
| PSA | 46.61000 |
| LogP | 0.27 |
| Vapour density | 4.9 (vs air) |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.574 |
| InChIKey | ZXNRTKGTQJPIJK-UHFFFAOYSA-N |
| SMILES | COc1ccc(C(=O)N2CCCC2=O)cc1 |
| Storage condition | Store at RT |
| Water Solubility | Reacts |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | UY5781900 |
|
~82% 
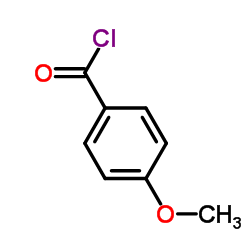
Aniracetam CAS#:72432-10-1 |
| Literature: Karimi; Kihlberg; Langstroem Journal of the Chemical Society. Perkin Transactions 1, 2001 , # 13 p. 1528 - 1531 |
|
~% 
Aniracetam CAS#:72432-10-1 |
| Literature: US4369139 A1, ; |
|
~39% 
Aniracetam CAS#:72432-10-1 |
| Literature: Zhang, Jian; Hong, Soon Hyeok Organic Letters, 2012 , vol. 14, # 17 p. 4646 - 4649 |
|
~% 
Aniracetam CAS#:72432-10-1 |
| Literature: US4369139 A1, ; |
|
~% 
Aniracetam CAS#:72432-10-1 |
| Literature: US4369139 A1, ; |
|
~70% 
Aniracetam CAS#:72432-10-1 |
| Literature: Blay, Gonzalo; Cardona, Luz; Garcia, Begona; Garcia, Cristina L.; Pedro, Jose R. Tetrahedron Letters, 1997 , vol. 38, # 47 p. 8257 - 8260 |
|
~% 
Aniracetam CAS#:72432-10-1 |
| Literature: US4369139 A1, ; |
|
~% 
Aniracetam CAS#:72432-10-1 |
| Literature: US4369139 A1, ; |
| Precursor 9 | |
|---|---|
| DownStream 3 | |
|
Piracetam defines a new binding site for allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors.
J. Med. Chem. 53 , 2197-203, (2010) Glutamate receptors are the most prevalent excitatory neurotransmitter receptors in the vertebrate central nervous system and are important potential drug targets for cognitive enhancement and the tre... |
|
|
Glutamate Receptors within the Mesolimbic Dopamine System Mediate Alcohol Relapse Behavior.
J. Neurosci. 35 , 15523-38, (2015) Glutamatergic input within the mesolimbic dopamine (DA) pathway plays a critical role in the development of addictive behavior. Although this is well established for some drugs of abuse, it is not kno... |
|
|
Development and validation of a liquid chromatographic method for the simultaneous determination of aniracetam and its related substances in the bulk drug and a tablet formulation.
J. Pharm. Biomed. Anal. 56(3) , 615-22, (2011) Simultaneous determination of aniracetam and its related impurities (2-pyrrolidinone, p-anisic acid, 4-p-anisamidobutyric acid and (p-anisoyl)-4-methyl-2-pyrrolidinone) was accomplished in the bulk dr... |
| MFCD00153767 |
| Ro 13-5057 |
| Aniracetam |
| 1-p-Anisoyl-2-pyrrolidinone |
| Sarpul |
| 1-(4-Methoxybenzoyl)pyrrolidin-2-one |
| Ampamet |
| Draganon |
| 1-[(4-Methoxyphenyl)carbonyl]pyrrolidin-2-on |
| 2-Pyrrolidinone, 1-(4-methoxybenzoyl)- |
| 1-anisoyl-2-pyrrolidinone |
| 1-(4-Methoxybenzoyl)-2-pyrrolidinone |
| 1-[(4-Methoxyphenyl)carbonyl]pyrrolidin-2-one |

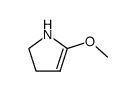


![4-[(4-Methoxybenzoyl)amino]butanoic acid structure](https://image.chemsrc.com/caspic/032/72432-14-5.png)
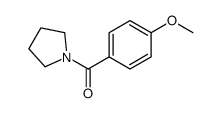
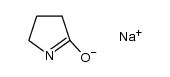
 CAS#:100-09-4
CAS#:100-09-4