| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
2-Pyrrolidinone
CAS:616-45-5 |
|
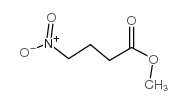 |
methyl 4-nitrobutyrate
CAS:13013-02-0 |