Structure-activity relationships of some pyridine, piperidine, and pyrrolidine analogues for enhancing and inhibiting the binding of (+/-)-[3H]nicotine to the rat brain P2 preparation.
J W Sloan, W R Martin, R Hook, J Hernandez
Index: J. Med. Chem. 28(9) , 1245-51, (1985)
Full Text: HTML
Abstract
Previous studies have shown that (+/-)-[3H]nicotine binds to multiple sites in the rat brain P2 preparation. Using a series of pyridine, piperidine and pyrrolidine analogues, the present studies identified drugs with specificity for a separate up-regulatory site that increases the density of nicotine binding at another site. Of these compounds, (+/-)-2-methylpiperidine was the most specific. Some compounds inhibited without enhancing (+/-)-[3H]nicotine binding, but none bound with the very high affinity exhibited by nicotine and none could be classified as specific in inhibiting binding at a specific site. Structural changes in the 1- and 2-positions of pyridine and piperidine appear to be important for conferring specificity for the up-regulatory site whereas 3-position changes may be important for binding specificity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
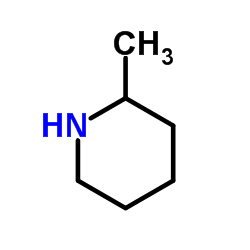 |
2-Pipecoline
CAS:109-05-7 |
C6H13N |
|
C-2 arylation of piperidines through directed transition-met...
2010-11-22 [Chemistry 16 , 13063-13067, (2010)] |
|
Gold-catalyzed efficient synthesis of azepan-4-ones via a tw...
2010-05-21 [Chem. Commun. (Camb.) 46 , 3351-3353, (2010)] |
|
Acetoacetanilides as masked isocyanates: facile and efficien...
2010-10-01 [Org. Lett. 12 , 4220-4223, (2010)] |
|
Synthesis of 2-aminobenzoxazoles using tetramethyl orthocarb...
2010-11-19 [J. Org. Chem. 75 , 7942-7945, (2010)] |
|
Design and synthesis of novel Gefitinib analogues with impro...
2010-06-01 [Bioorg. Med. Chem. 18 , 3812-3822, (2010)] |