Antitumor and antimicrobial activities of some hetero aromatic benzofurans derived from naturally occurring visnagin.
Sally S El-Nakkady, Hanaa F Roaiah, Weam S El-Serwy, Abdel Mohsen Soliman, Sherein I Abd El-Moez, Adel A H Abdel-Rahman
Index: Acta Pol. Pharm. 69(4) , 645-55, (2012)
Full Text: HTML
Abstract
Bromination of visnaginone (1) yielded the dibromo derivative (2), which upon methylation with methyl iodide gave 1-(2,7-dibromo-4,6-dimethoxybenzofuran-5-yl) ethanone (3). Compound (3) reacted with dimethylformamide dimethylacetal to give (4). The reaction of (3) with aromatic aldehydes namely (vanillin, benzaldehyde and 3-anisaldehyde) in ammonium acetate, malononitrile and/or butyric cyanoanhydride gave the 2-amino substituted nicotinonitriles (5a-c) and the 2-hydroxyl substituted nicotinonitriles (7a-c), respectively, while in piperidine gave (E)-1-(2,7-dibromo-4,6-dimethoxybenzofuran-5-yl)-3-(substituted)prop-2-en-l-one (11a-c). (5a) was hydrolyzed with sulfuric acid on cold to give the nicotinic acid derivative (6a). When compound (3) reacted with hydrazines and aromatic amines, it gave the Schiff bases (8a,b) and (10a,b), respectively. (8b) reacted with thioglycolic acid to give the thiazolidin-4-one (9b). When (11a-c) reacted with thiourea, it gave the pyrimidine derivatives (12a-c). (11a,b) also reacted with butyric cyanoanhydride and hydroxylamine hydrochloride to give (13a,b) and (15a,b), respectively. When the carboxylate (13a) was treated with 2,4-dinitroaniline, it gave the carboxamide (14a). Compounds (11b,c) reacted with hydrazine derivatives (hydrazine hydrate and phenylhydrazine) yielding the substituted pyrazole derivatives (16b,c) and (17b,c), respectively. All the structures of the synthesized compounds were elucidated by elemental analyses and spectral data. The newly synthesized benzofuran compounds showed a strong to moderate cytotoxicity against liver HEPG2 cancer cell line compared to 5-fluorouracil and doxorubicin (the anticancer agents). Compounds (2, 6a, 13a, 14a, 16c and 17b) were the most active compounds in descending order. The synthesized compounds were also tested for their antimicrobial activity. Compound (10b) showed the highest activity against all the tested strains followed by 6, 10a, 5a, 8b and 7a in descending order.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
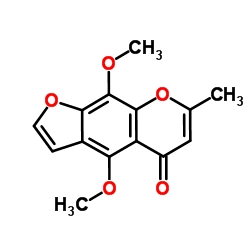 |
Khellin
CAS:82-02-0 |
C14H12O5 |
|
Validated HPLC and HPTLC methods for simultaneous determinat...
2013-03-01 [J. Chromatogr. Sci. 51(3) , 258-65, (2013)] |
|
F420H2-dependent degradation of aflatoxin and other furanoco...
2012-01-01 [PLoS ONE 7(2) , e30114, (2012)] |
|
A case study to evaluate the treatment of vitiligo with khel...
2003-01-01 [Eur. J. Dermatol. 13(5) , 474-7, (2003)] |
|
Application of 1D BIRD or X-filtered DEPT long-range C-C rel...
2006-04-01 [Magn. Reson. Chem. 44(4) , 475-80, (2006)] |
|
Computational study of khellin excited states and photobindi...
2009-08-01 [Photochem. Photobiol. Sci. 8(8) , 1179-86, (2009)] |