Synthesis, antiproliferative activity, acute toxicity and assessment of the antiandrogenic activities of new androstane derivatives.
Neelima Dhingra, T R Bhardwaj, Neeraj Mehta, Tapas Mukhopadhyay, Ashok Kumar, Manoj Kumar
Index: Arch. Pharm. Res. 34(7) , 1055-63, (2011)
Full Text: HTML
Abstract
A number of 17-oxo-5-androsten-3β-yl esters (9a-9f) and 3β-alkoxy-5-androsten-17-ones (11a-11e) were synthesized from commercially available (25R)-5-spirosten-3β-ol (Diosgenin) (4) as starting material. The synthesized compounds were evaluated for their antiproliferative activity against the prostate-specific cancer cell line DU-145, acute toxicity and effect on serum androgen levels, and compared with finasteride as positive control. Some of the compounds exhibited better cytotoxicity and antiandrogenic activity than the reference control. The detailed synthesis, spectroscopic data and biological activity of the synthesized compounds are reported.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
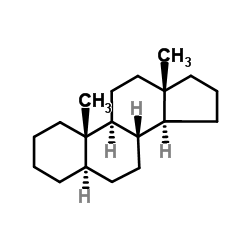 |
5a-androstane
CAS:438-22-2 |
C19H32 |
|
Potent and selective steroidal inhibitors of 17beta-hydroxys...
2009-12-10 [J. Med. Chem. 52 , 7488-502, (2009)] |
|
Novel and efficient synthesis and antifungal evaluation of 2...
2008-01-01 [Bioorg. Med. Chem. Lett. 20 , 7372-5, (2010)] |
|
[Bioconversion of C19- and C21-steroids with parent and muta...
2010-01-01 [Prikl. Biokhim. Mikrobiol. 46(2) , 212-20, (2010)] |
|
Synthesis, characterization and biological evaluation of som...
2011-01-01 [Steroids 76(7) , 709-23, (2011)] |
|
Synthesis and antitumor activity of new D-seco and D-homo an...
2009-11-01 [Steroids 74(12) , 983-8, (2009)] |