5a-androstane
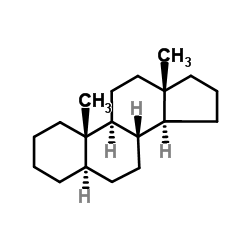
5a-androstane structure
|
Common Name | 5a-androstane | ||
|---|---|---|---|---|
| CAS Number | 438-22-2 | Molecular Weight | 260.457 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 336.0±9.0 °C at 760 mmHg | |
| Molecular Formula | C19H32 | Melting Point | 78-82 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 145.9±12.3 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
|
Potent and selective steroidal inhibitors of 17beta-hydroxysteroid dehydrogenase type 7, an enzyme that catalyzes the reduction of the key hormones estrone and dihydrotestosterone.
J. Med. Chem. 52 , 7488-502, (2009) 17beta-Hydroxysteroid dehydrogenase type 7 (17beta-HSD7) catalyzes the reduction of estrone (E(1)) into estradiol (E(2)) and of dihydrotestosterone (DHT) into 5alpha-androstane-3beta,17beta-diol (3beta-diol), therefore modulating the level of mitogenic estrog... |
|
|
Novel and efficient synthesis and antifungal evaluation of 2,3-functionalized cholestane and androstane derivatives
Bioorg. Med. Chem. Lett. 20 , 7372-5, (2010) Synthetic modifications of cholesterol and other traditional steroid molecules have become a promising area for the exploration and development of novel antifungal agents, especially with respect to the development of fatty-acid esters of steroids. In additio... |
|
|
[Bioconversion of C19- and C21-steroids with parent and mutant strains of Curvularia lunata].
Prikl. Biokhim. Mikrobiol. 46(2) , 212-20, (2010) Regio- and stereospecificity of microbial hydroxylation was studied at the transformation of 3-keto-4-ene steroids of androstane and pregnane series by the filamentous fungus of Curvularia lunata VKMF-644. The products of the transformations were isolated by ... |
|
|
Synthesis, antiproliferative activity, acute toxicity and assessment of the antiandrogenic activities of new androstane derivatives.
Arch. Pharm. Res. 34(7) , 1055-63, (2011) A number of 17-oxo-5-androsten-3β-yl esters (9a-9f) and 3β-alkoxy-5-androsten-17-ones (11a-11e) were synthesized from commercially available (25R)-5-spirosten-3β-ol (Diosgenin) (4) as starting material. The synthesized compounds were evaluated for their antip... |
|
|
Synthesis, characterization and biological evaluation of some 16E-arylidene androstane derivatives as potential anticancer agents
Steroids 76(7) , 709-23, (2011) Graphical abstract . A series of new 16E-arylidene androstane derivatives were synthesized and evaluated for their anticancer activities against four cancer cell lines SW480, A549, HepG2 and HeLa in vitro. |
|
|
Synthesis and antitumor activity of new D-seco and D-homo androstane derivatives.
Steroids 74(12) , 983-8, (2009) Starting from 3beta-hydroxy-17-oxo-16,17-secoandrost-5-ene-16-nitrile (1), the new 16,17-secoandrostane derivatives 4-9 were synthesized. On the other hand, 3beta-hydroxy-17-oxa-D-homoandrost-5-ene-16-one (10) yielded the new d-homo derivatives 12, 13 and 15.... |
|
|
Steroid 9alpha-hydroxylation during testosterone degradation by resting rhodococcus equi cells.
Arch. Pharm. (Weinheim) 340(4) , 209-14, (2007) The conversion pathway of testosterone to androst-4-ene-3,17-dione and 9alpha-hydroxy androstane metabolites, 9alpha-hydroxyandrost-4-ene-3,17-dione and 9alpha,17beta-dihydroxyandrost-4-en-3-one was proposed for the ring degradation in steroids by a minimal l... |
|
|
Synthesis of [19- 2H3]-analogs of dehydroepiandrosterone and pregnenolone and their sulfates.
Steroids 69(3) , 161-71, (2004) Deuterated analogs of pregnenolone and pregnenolone sulfate with three atoms of deuterium in position 19 were prepared. The synthetic approach was developed on derivatives of dehydroepiandrosterone, where initial intermediates were well characterized, and the... |
|
|
Synthesis of 3-methyl-3-hydroxy-6-oxo-androstane derivatives.
Steroids 69(10) , 605-12, (2004) 3alpha,17beta-Dihydroxy-3beta-methyl-5alpha-androstan-6-one (1) and 3beta,17beta-dihydroxy-3alpha-methyl-5alpha-androstan-6-one (13) were prepared by the reaction of methylmagnesium bromide with the 3-ketosteroids. Structures and configurations in position 3 ... |
|
|
The synthesis of androstane brassinosteroid analogues with alpha-azido acid ester groups in position 17beta.
Steroids 75(12) , 1005-10, (2010) Androstane brassinosteroid analogues with alpha-azido acid ester groups in position 17beta were synthesized from 2alpha,3alpha,17beta-trihydroxy-5alpha-androstan-6-one after the protection of the 2alpha,3alpha-diols upon treatment with the corresponding alpha... |