| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Resveratrol
CAS:501-36-0 |
|
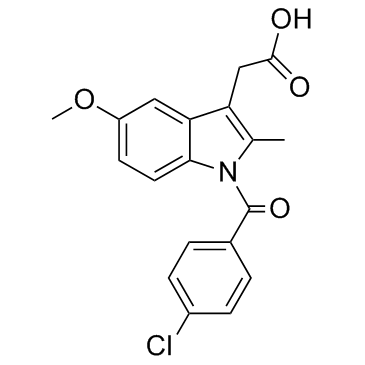 |
Indometacin
CAS:53-86-1 |
|
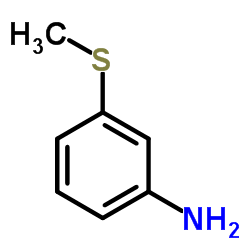 |
3-(Methylmercapto)aniline
CAS:1783-81-9 |