CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
NL3500000
-
CHEMICAL NAME :
-
Indole-3-acetic acid, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-
-
CAS REGISTRY NUMBER :
-
53-86-1
-
BEILSTEIN REFERENCE NO. :
-
0497341
-
LAST UPDATED :
-
199703
-
DATA ITEMS CITED :
-
125
-
MOLECULAR FORMULA :
-
C19-H16-Cl-N-O4
-
MOLECULAR WEIGHT :
-
357.81
-
WISWESSER LINE NOTATION :
-
T56 BNJ BVR CG& C1 D1VQ GO1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
4286 ug/kg/2D-I
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, other (after systemic exposure)
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
15 mg/kg/2W-I
-
TOXIC EFFECTS :
-
Blood - aplastic anemia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - infant
-
DOSE/DURATION :
-
400 ug/kg/2D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - necrotic changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
2098 ug/kg/1D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Kidney, Ureter, Bladder - urine volume decreased
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
22500 ug/kg/3W-I
-
TOXIC EFFECTS :
-
Liver - hepatitis, fibrous (cirrhosis, post-necrotic scarring) Liver - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
113 mg/kg/8W-I
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Gastrointestinal - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - infant
-
DOSE/DURATION :
-
200 ug/kg
-
TOXIC EFFECTS :
-
Blood - hemorrhage
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
499 mg/kg/87W-I
-
TOXIC EFFECTS :
-
Behavioral - stiffness Kidney, Ureter, Bladder - hematuria
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Rectal
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
2586 mg/kg/3.5Y-I
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - retinal changes (pigmentary depositions, retinitis, other)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Multiple routes
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
3557 mg/kg/5Y-I
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - retinal changes (pigmentary depositions, retinitis, other)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2420 ug/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>250 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
13 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
12 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
21 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
26300 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Rectal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
31900 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
11841 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
18300 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
30 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
18200 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
160 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
320 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
20200 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
135 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
>100 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - excitement
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
143 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
180 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
81 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - species unspecified
-
DOSE/DURATION :
-
8 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
33 mg/kg/11D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Gastrointestinal - ulceration or bleeding from duodenum Gastrointestinal - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
168 mg/kg/4W-C
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - changes in lung weight Endocrine - changes in spleen weight Blood - normocytic anemia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
273 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Blood - normocytic anemia Biochemical - Metabolism (Intermediary) - other proteins Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1820 mg/kg/52W-C
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from small intestine Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
210 mg/kg/35D-C
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Endocrine - other changes Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
650 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Gastrointestinal - ulceration or bleeding from small intestine Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
105 mg/kg/21D-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - changes in bladder weight Related to Chronic Data - changes in ovarian weight
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
24 mg/kg/4D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
150 mg/kg/5W-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - other changes Blood - changes in spleen Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
90 mg/kg/90D-C
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Gastrointestinal - ulceration or bleeding from small intestine Gastrointestinal - hypermotility, diarrhea
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
350 mg/kg/5W-I
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from large intestine Kidney, Ureter, Bladder - other changes in urine composition Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
135 mg/kg/90D-C
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Blood - changes in leukocyte (WBC) count
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - species unspecified
-
DOSE/DURATION :
-
168 mg/kg/4W-I
-
TOXIC EFFECTS :
-
Gastrointestinal - necrotic changes Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Nutritional and Gross Metabolic - changes in chlorine
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
644 mg/kg/92W-C
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Skin and Appendages - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1500 ug/kg
-
SEX/DURATION :
-
female 36 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - respiratory system Reproductive - Effects on Newborn - other neonatal measures or effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1500 mg/kg
-
SEX/DURATION :
-
female 27 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
12 mg/kg
-
SEX/DURATION :
-
female 33-34 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
525 mg/kg
-
SEX/DURATION :
-
female 16-32 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Maternal Effects - other effects Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
18 mg/kg
-
SEX/DURATION :
-
female 33-34 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - homeostasis
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
6 mg/kg
-
SEX/DURATION :
-
female 39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
4 mg/kg
-
SEX/DURATION :
-
female 10 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 28 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system Reproductive - Specific Developmental Abnormalities - homeostasis
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
12 mg/kg
-
SEX/DURATION :
-
female 32 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
14 mg/kg
-
SEX/DURATION :
-
female 34 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - other neonatal measures or effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Multiple routes
-
DOSE :
-
22 mg/kg
-
SEX/DURATION :
-
female 33 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Effects on Newborn - Apgar score (human only)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Rectal
-
DOSE :
-
12 mg/kg
-
SEX/DURATION :
-
female 28 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - blood and lymphatic systems (including spleen and marrow) Reproductive - Specific Developmental Abnormalities - gastrointestinal system Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 mg/kg
-
SEX/DURATION :
-
female 21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
300 ug/kg
-
SEX/DURATION :
-
female 21-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - live birth index (measured after birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10 mg/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - respiratory system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
12 mg/kg
-
SEX/DURATION :
-
female 5-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 1-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2500 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
28 mg/kg
-
SEX/DURATION :
-
male 7 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
4150 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 5 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2500 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
12 mg/kg
-
SEX/DURATION :
-
female 3-4 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
9 mg/kg
-
SEX/DURATION :
-
female 9 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
3 mg/kg
-
SEX/DURATION :
-
female 19-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - respiratory system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
3 mg/kg
-
SEX/DURATION :
-
female 20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intrauterine
-
DOSE :
-
500 ug/kg
-
SEX/DURATION :
-
female 4 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravaginal
-
DOSE :
-
125 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4 mg/kg
-
SEX/DURATION :
-
female 13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
9 mg/kg
-
SEX/DURATION :
-
female 2 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
40 mg/kg
-
SEX/DURATION :
-
female 8-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - body wall
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
40 mg/kg
-
SEX/DURATION :
-
female 8-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
8 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
8 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
496 mg/kg
-
SEX/DURATION :
-
female 21-26 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - physical
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
747 mg/kg
-
SEX/DURATION :
-
female 21-26 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
6 mg/kg
-
SEX/DURATION :
-
female 2 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
96 mg/kg
-
SEX/DURATION :
-
female 20-31 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10 mg/kg
-
SEX/DURATION :
-
female 29 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
6 mg/kg
-
SEX/DURATION :
-
female 2 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1590 ug/kg
-
SEX/DURATION :
-
female 6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - maternal-fetal exchange
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
48 mg/kg
-
SEX/DURATION :
-
female 23-28 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
32 mg/kg
-
SEX/DURATION :
-
female 5-6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
24 mg/kg
-
SEX/DURATION :
-
female 4-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
64 mg/kg
-
SEX/DURATION :
-
female 9-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 26-29 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
148 mg/kg
-
SEX/DURATION :
-
female 1-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
7500 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
5500 ug/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 24-26 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - respiratory system Reproductive - Effects on Newborn - biochemical and metabolic
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
2500 ng/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
160 mg/kg
-
SEX/DURATION :
-
female 16 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
240 mg/kg
-
SEX/DURATION :
-
female 12 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intrauterine
-
DOSE :
-
66640 ug/kg
-
SEX/DURATION :
-
female 9 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
144 mg/kg
-
SEX/DURATION :
-
female 18 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
40 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 mg/kg
-
SEX/DURATION :
-
female 21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
Mutation test systems - not otherwise specified
-
TYPE OF TEST :
-
Dominant lethal test
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Sperm Morphology
MUTATION DATA
-
TEST SYSTEM :
-
Rodent - mouse
-
DOSE/DURATION :
-
60 mg/kg/5D (Continuous)
-
REFERENCE :
-
MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 188,343,1987 *** REVIEWS *** TOXICOLOGY REVIEW CTOXAO Clinical Toxicology. (New York, NY) V.1-18, 1968-81. For publisher information, see JTCTDW. Volume(issue)/page/year: 17,85,1980 TOXICOLOGY REVIEW REPTED Reproductive Toxicology. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.1- 1987- Volume(issue)/page/year: 9,7,1995 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X4091 No. of Facilities: 123 (estimated) No. of Industries: 2 No. of Occupations: 10 No. of Employees: 5082 (estimated) No. of Female Employees: 2130 (estimated)
|
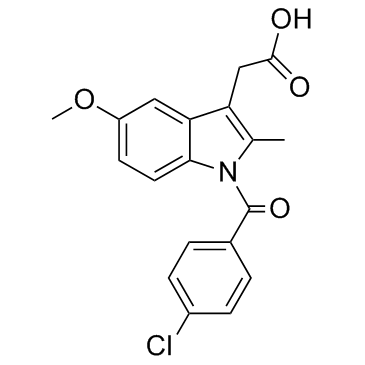


 CAS#:19501-58-7
CAS#:19501-58-7 CAS#:122-01-0
CAS#:122-01-0 CAS#:123-76-2
CAS#:123-76-2![2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetaldehyde Structure](https://image.chemsrc.com/caspic/211/24438-49-1.png) CAS#:24438-49-1
CAS#:24438-49-1 CAS#:16401-99-3
CAS#:16401-99-3![4-METHYL-[1,1-BIPHENYL]-4-CARBOXYLICACID Structure](https://image.chemsrc.com/caspic/411/17536-38-8.png) CAS#:17536-38-8
CAS#:17536-38-8 CAS#:591-12-8
CAS#:591-12-8 CAS#:16390-07-1
CAS#:16390-07-1 CAS#:873657-14-8
CAS#:873657-14-8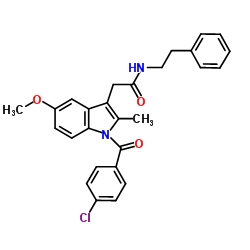 CAS#:261766-32-9
CAS#:261766-32-9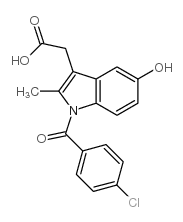 CAS#:2504-32-7
CAS#:2504-32-7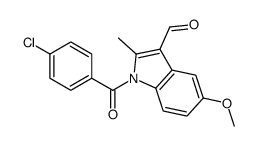 CAS#:23694-51-1
CAS#:23694-51-1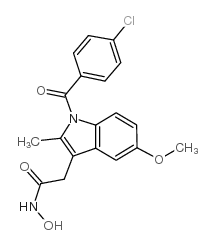 CAS#:27035-30-9
CAS#:27035-30-9 CAS#:2854-32-2
CAS#:2854-32-2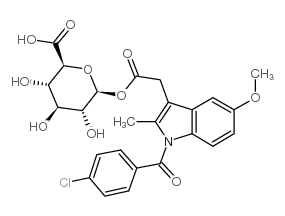 CAS#:75523-11-4
CAS#:75523-11-4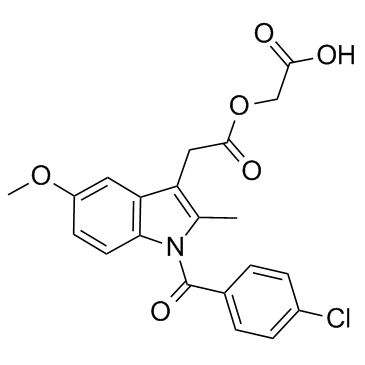 CAS#:53164-05-9
CAS#:53164-05-9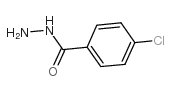 CAS#:536-40-3
CAS#:536-40-3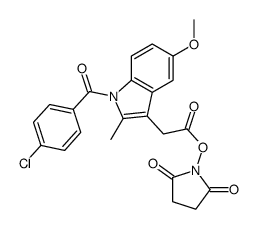 CAS#:104425-42-5
CAS#:104425-42-5 CAS#:1601-19-0
CAS#:1601-19-0
