| Structure | Name/CAS No. | Articles |
|---|---|---|
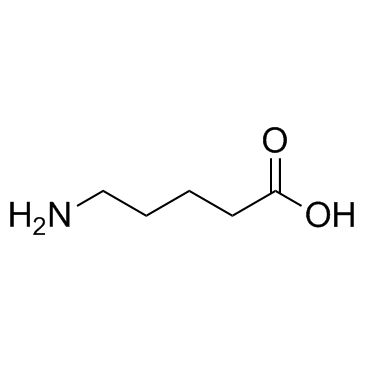 |
5-Aminovaleric acid
CAS:660-88-8 |
|
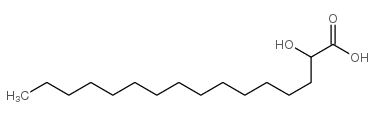 |
2-hydroxy Palmitic Acid
CAS:764-67-0 |
|
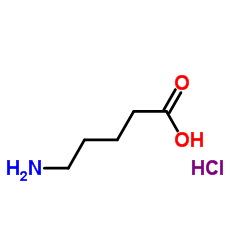 |
4-carboxybutan-1-aminium chloride
CAS:627-95-2 |