5-Aminovaleric acid
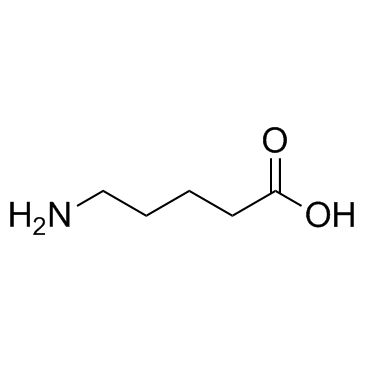
5-Aminovaleric acid structure
|
Common Name | 5-Aminovaleric acid | ||
|---|---|---|---|---|
| CAS Number | 660-88-8 | Molecular Weight | 117.146 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 247.5±23.0 °C at 760 mmHg | |
| Molecular Formula | C5H11NO2 | Melting Point | 158-161 °C(lit.) | |
| MSDS | USA | Flash Point | 103.5±22.6 °C | |
|
Peptide dimethylation: fragmentation control via distancing the dimethylamino group.
J. Am. Soc. Mass Spectrom. 25(10) , 1694-704, (2014) Direct reductive methylation of peptides is a common method for quantitative proteomics. It is an active derivatization technique; with participation of the dimethylamino group, the derivatized peptides preferentially release intense a1 ions. The advantageous... |
|
|
Detection of autosomal dominant polycystic kidney disease by NMR spectroscopic fingerprinting of urine.
Kidney Int. 79(11) , 1244-53, (2011) Autosomal dominant polycystic kidney disease (ADPKD) is a frequent cause of kidney failure; however, urinary biomarkers for the disease are lacking. In a step towards identifying such markers, we used multidimensional-multinuclear nuclear magnetic resonance (... |
|
|
Highly sensitive GC/MS/MS method for quantitation of amino and nonamino organic acids.
Anal. Chem. 83(7) , 2705-11, (2011) Metabolite profiling methods are important tools for measurement of metabolite pools in biological systems. While most metabolite profiling methods report relative intensities or depend on a few internal standards representing all metabolites, the ultimate re... |
|
|
Differential 12C-/13C-isotope dansylation labeling and fast liquid chromatography/mass spectrometry for absolute and relative quantification of the metabolome.
Anal. Chem. 81(10) , 3919-32, (2009) We report a new quantitative metabolome profiling technique based on differential (12)C-/(13)C-isotope dansylation labeling of metabolites, fast liquid chromatography (LC) separation and electrospray ionization Fourier-transform ion cyclotron resonance mass s... |
|
|
Development of imidazole alkanoic acids as mGAT3 selective GABA uptake inhibitors.
Eur. J. Med. Chem. 46 , 1483-98, (2011) A new series of potential GABA uptake inhibitors starting from of 1H-imidazol-4-ylacetic acid with the carboxylic acid side chain originating from different positions and varying in length have been synthesized and tested for the inhibitory potency at the fou... |
|
|
Solid-state NMR analyses reveal the structure dependence of the molecular dynamics for ω-amino acids.
J. Phys. Chem. B 116(7) , 2096-103, (2012) The molecular dynamics of metabolites is structure dependent and vitally important for the interactive functions in their potential applications as natural materials. To understand the relationship between molecular structure and dynamics, the molecular motio... |
|
|
Metabolic engineering of Escherichia coli for the production of 5-aminovalerate and glutarate as C5 platform chemicals.
Metab. Eng. 16 , 42-7, (2013) 5-Aminovalerate (5AVA) is the precursor of valerolactam, a potential building block for producing nylon 5, and is a C5 platform chemical for synthesizing 5-hydroxyvalerate, glutarate, and 1,5-pentanediol. Escherichia coli was metabolically engineered for the ... |
|
|
Novel gamma-aminobutyric acid rho1 receptor antagonists; synthesis, pharmacological activity and structure-activity relationships.
J. Med. Chem. 51 , 3825-40, (2008) Gamma-aminobutyric acid (GABA) analogues based on 4-amino-cyclopent-1-enyl phosphinic acid ( 34- 42) and 3-aminocyclobutane phosphinic acids ( 51, 52, 56, 57) were investigated in order to obtain selective homomeric rho 1 GABA C receptor antagonists. The effe... |
|
|
Heterologous expression, purification, and characterization of an l-ornithine N(5)-hydroxylase involved in pyoverdine siderophore biosynthesis in Pseudomonas aeruginosa.
J. Bacteriol. 188(20) , 7205-10, (2006) Pseudomonas aeruginosa is an opportunistic pathogen that produces the siderophore pyoverdine, which enables it to acquire the essential nutrient iron from its host. Formation of the iron-chelating hydroxamate functional group in pyoverdine requires the enzyme... |
|
|
Transient state kinetic investigation of 5-aminolevulinate synthase reaction mechanism.
J. Biol. Chem. 277(47) , 44660-9, (2002) 5-Aminolevulinate synthase (ALAS), a pyridoxal 5'-phosphate-dependent enzyme, catalyzes the first, and regulatory, step of the heme biosynthetic pathway in nonplant eukaryotes and some bacteria. 5-Aminolevulinate synthase is a dimeric protein having an ordere... |