| Structure | Name/CAS No. | Articles |
|---|---|---|
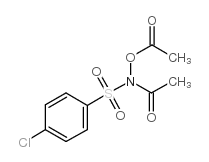 |
n-acetyl-n-acetoxy-4-chlorobenzenesulfonamide
CAS:142867-52-5 |
|
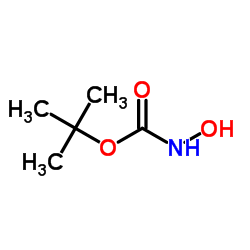 |
tert-Butyl hydroxycarbamate
CAS:36016-38-3 |