tert-Butyl hydroxycarbamate
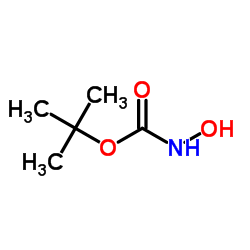
tert-Butyl hydroxycarbamate structure
|
Common Name | tert-Butyl hydroxycarbamate | ||
|---|---|---|---|---|
| CAS Number | 36016-38-3 | Molecular Weight | 133.146 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 250.4±9.0 °C at 760 mmHg | |
| Molecular Formula | C5H11NO3 | Melting Point | 53-55 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 105.2±18.7 °C | |
| Name | tert-Butyl N-hydroxycarbamate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 250.4±9.0 °C at 760 mmHg |
| Melting Point | 53-55 °C(lit.) |
| Molecular Formula | C5H11NO3 |
| Molecular Weight | 133.146 |
| Flash Point | 105.2±18.7 °C |
| Exact Mass | 133.073898 |
| PSA | 58.56000 |
| LogP | 0.82 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.443 |
| InChIKey | DRDVJQOGFWAVLH-UHFFFAOYSA-N |
| SMILES | CC(C)(C)OC(=O)NO |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29280090 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
N-O chemistry for antibiotics: discovery of N-alkyl-N-(pyridin-2-yl)hydroxylamine scaffolds as selective antibacterial agents using nitroso Diels-Alder and ene chemistry.
J. Med. Chem. 54 , 6843-58, (2011) The discovery, syntheses, and structure-activity relationships (SAR) of a new family of heterocyclic antibacterial compounds based on N-alkyl-N-(pyridin-2-yl)hydroxylamine scaffolds are described. A s... |
|
|
Prodrugs of nitroxyl as inhibitors of aldehyde dehydrogenase.
J. Med. Chem. 35 , 3648, (1992) In the preceding paper, analogs of chlorpropamide with an OMe substituent on the sulfonamide nitrogen were shown to inhibit aldehyde dehydrogenase (AlDH), and it was postulated that these compounds we... |
|
|
Reactions of nitroso hetero-Diels-Alder cycloadducts with azides: stereoselective formation of triazolines and aziridines.
J. Org. Chem. 72 , 3929, (2007) The addition of azides to acylnitroso hetero-Diels-Alder cycloadducts derived from cyclopentadiene affords exo-triazolines in excellent yield. The reaction is greatly affected by the level of alkene s... |
| (tert-butyl)oxycarbohydroxamic acid |
| N-(tert-Butoxycarbonyl)hydroxylamine |
| tert-butyl N-hydroxy-carbamate |
| N-tert-Butoxycarbonylhydroxylamine |
| MFCD00002107 |
| tert-Butyl hydroxycarbamate |
| t-Butyl N-hydroxycarbamate |
| EINECS 252-836-2 |
| N-Boc-hydroxylamine |
| 1,1-dimethylethyl N-hydroxycarbamate |
| N-Hydroxycarbamic Acid tert-Butyl Ester |
| 2-Methyl-2-propanyl hydroxycarbamate |
| N-(t-Butoxycarbonyl)-hydroxylamine |
| Carbamic acid, N-hydroxy-, 1,1-dimethylethyl ester |
 CAS#:24424-99-5
CAS#:24424-99-5 CAS#:1070-19-5
CAS#:1070-19-5 CAS#:5470-11-1
CAS#:5470-11-1![bis[(2-methylpropan-2-yl)oxycarbonyl] carbonate Structure](https://image.chemsrc.com/caspic/037/24424-95-1.png) CAS#:24424-95-1
CAS#:24424-95-1 CAS#:870-46-2
CAS#:870-46-2![tert-butyl 1,4-dioxo-1H-benzo[d][1,2]oxazine-3(4H)-carboxylate Structure](https://image.chemsrc.com/caspic/242/31583-38-7.png) CAS#:31583-38-7
CAS#:31583-38-7 CAS#:105340-85-0
CAS#:105340-85-0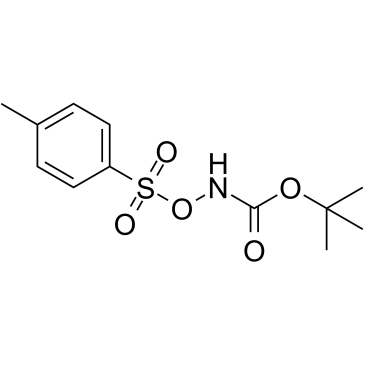 CAS#:105838-14-0
CAS#:105838-14-0![TERT-BUTYL 2-OXA-3-AZABICYCLO[2.2.2]OCT-5-ENE-3-CARBOXYLATE structure](https://image.chemsrc.com/caspic/148/110590-29-9.png) CAS#:110590-29-9
CAS#:110590-29-9![tert-Butyl [(mesitylsulfonyl)oxy]carbamate structure](https://image.chemsrc.com/caspic/376/36016-39-4.png) CAS#:36016-39-4
CAS#:36016-39-4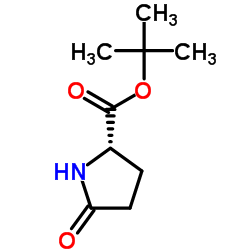 CAS#:35418-16-7
CAS#:35418-16-7 CAS#:144751-62-2
CAS#:144751-62-2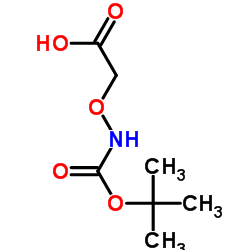 CAS#:42989-85-5
CAS#:42989-85-5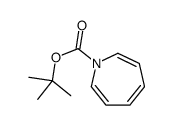 CAS#:20646-60-0
CAS#:20646-60-0 CAS#:19689-97-5
CAS#:19689-97-5 CAS#:85006-25-3
CAS#:85006-25-3 CAS#:66605-57-0
CAS#:66605-57-0
