tert-Butyl hydroxycarbamate
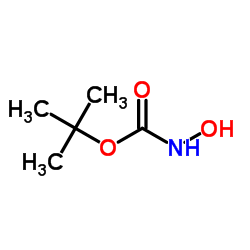
tert-Butyl hydroxycarbamate structure
|
Common Name | tert-Butyl hydroxycarbamate | ||
|---|---|---|---|---|
| CAS Number | 36016-38-3 | Molecular Weight | 133.146 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 250.4±9.0 °C at 760 mmHg | |
| Molecular Formula | C5H11NO3 | Melting Point | 53-55 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 105.2±18.7 °C | |
|
N-O chemistry for antibiotics: discovery of N-alkyl-N-(pyridin-2-yl)hydroxylamine scaffolds as selective antibacterial agents using nitroso Diels-Alder and ene chemistry.
J. Med. Chem. 54 , 6843-58, (2011) The discovery, syntheses, and structure-activity relationships (SAR) of a new family of heterocyclic antibacterial compounds based on N-alkyl-N-(pyridin-2-yl)hydroxylamine scaffolds are described. A structurally diverse library of ∼100 heterocyclic molecules ... |
|
|
Prodrugs of nitroxyl as inhibitors of aldehyde dehydrogenase.
J. Med. Chem. 35 , 3648, (1992) In the preceding paper, analogs of chlorpropamide with an OMe substituent on the sulfonamide nitrogen were shown to inhibit aldehyde dehydrogenase (AlDH), and it was postulated that these compounds were bioactivated by O-demethylation to release nitroxyl (HN ... |
|
|
Reactions of nitroso hetero-Diels-Alder cycloadducts with azides: stereoselective formation of triazolines and aziridines.
J. Org. Chem. 72 , 3929, (2007) The addition of azides to acylnitroso hetero-Diels-Alder cycloadducts derived from cyclopentadiene affords exo-triazolines in excellent yield. The reaction is greatly affected by the level of alkene strain, while sterically demanding azides do not hinder the ... |
|
|
D.S. Jones et al.
Tetrahedron Lett. 41 , 1531, (2000)
|
|
|
M. Adamczyk, R.E. Reddy
Synth. Commun. 31 , 579, (2001)
|
|
|
J.M. Altenburger et al.
Tetrahedron Lett. 33 , 5055, (1992)
|
|
|
S. Iwasa et al.
Tetrahedron Lett. 42 , 5897, (2001)
|
|
|
Tetrahedron Lett. 33 , 5055, (1992)
|
|
|
Synth. Commun. 22 , 2579, (1992)
|
|
|
Useful hydroxylamine derivatives for the synthesis of hydroxamic acids.
Tetrahedron Lett. 33(35) , 5055-5058, (1992)
|