| Structure | Name/CAS No. | Articles |
|---|---|---|
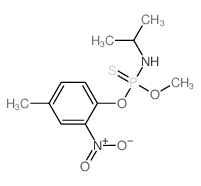 |
Amiprofos methyl
CAS:36001-88-4 |
|
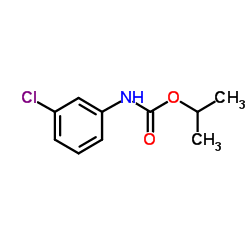 |
Chlorpropham
CAS:101-21-3 |