Bioorganic & Medicinal Chemistry Letters
2004-02-09
2-hydroxy-4-isopropylbenzaldehyde, a potent partial tyrosinase inhibitor.
Ken-ichi Nihei, Yoshiro Yamagiwa, Tadao Kamikawa, Isao Kubo
Index: Bioorg. Med. Chem. Lett. 14(3) , 681-3, (2004)
Full Text: HTML
Abstract
Chamaecin (2-hydroxy-4-isopropylbenzaldehyde) was synthesized and tested for its tyrosinase inhibitory activity. It partially inhibits the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) catalyzed by mushroom tyrosinase with an IC(50) of 2.3 microM. The inhibition kinetics analyzed by Dixon plots found that chamaecin is a mixed type inhibitor. This inhibition may come in part from its ability to form a Schiff base with a primary amino group in the enzyme.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
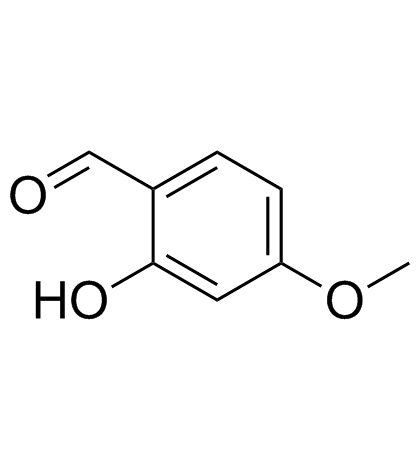 |
2-Hydroxy-4-methoxybenzaldehyde
CAS:673-22-3 |
C8H8O3 |
Related Articles:
More...
|
3D-QSAR and molecular docking studies of benzaldehyde thiose...
2007-03-01 [Bioorg. Med. Chem. 15 , 2006-15, (2007)] |
|
Flavoring extracts of Hemidesmus indicus roots and Vanilla p...
2013-09-01 [Plant Foods Hum. Nutr. 68(3) , 247-53, (2013)] |
|
Inhibition of Cancer Cell Proliferation and Antiradical Effe...
2015-06-01 [Phytother Res. 29 , 857-63, (2015)] |
|
Shikimate pathway modulates the elicitor-stimulated accumula...
2012-07-01 [Plant Physiol. Biochem. 56 , 104-8, (2012)] |
|
2-Hydroxy-4-methoxybenzaldehyde: a potent tyrosinase inhibit...
1999-02-01 [Planta Med. 65(1) , 19-22, (1999)] |