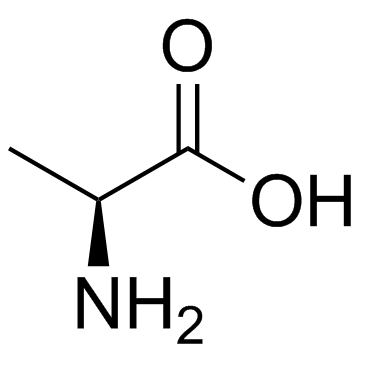
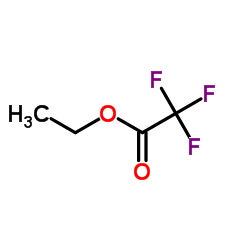
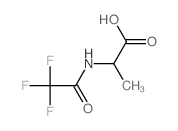
|
~89% |
|
~% |
|
~% |
|
~62% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~88% |
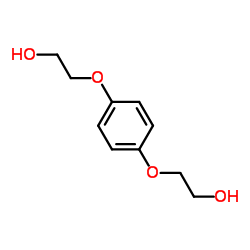
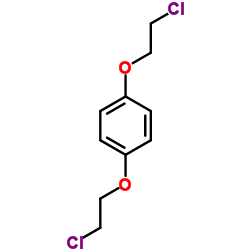
![Benzo[1,2-b:4,5-b']difuran,2,3,6,7-tetrahydro Structure](https://image.chemsrc.com/caspic/487/81926-24-1.png)
![(R)-(+)-N-trifluoroacetyl-1-(2,3,6,7-tetrahydrobenzo[1,2-b,4,5-b']difuran-4-yl)-2-aminopropane Structure](https://image.chemsrc.com/caspic/054/332012-04-1.png)


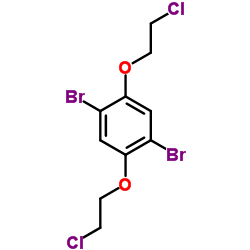

![(R)-(+)-N-trifluoroacetyl-2,3,6,7-tetrahydro-4-alanylbenzo[1,2-b,4,5-b']difuran Structure](https://image.chemsrc.com/caspic/140/332012-02-9.png)