On the metabolic activation of benz[a]acridine and benz[c]acridine by rat liver and lung microsomes.
J Jacob, A Schmoldt, W Kohbrok, G Raab, G Grimmer
Index: Cancer Lett. 16(3) , 297-306, (1982)
Full Text: HTML
Abstract
The metabolism of benz[a]- and benz[c]acridine by liver and lung microsomes from untreated, phenobarbital (PB)-treated and benzo[k]fluoranthene (BkF)-treated rats has been studied by gas chromatography/mass spectrometry (GC/MS). Epoxidation and hydrolysis of the epoxides to dihydrodiols were found to be the predominant pathways for all substrates. N-Oxidation is likely to occur in the case of benz[c]acridine. However, no unequivocal evidence could be obtained for the formation of the ultimate carcinogens--the t-3,4-dihydrodiol-1,2-epoxides--in case of both benz[a]- and benz[c]acridine. K-Region oxidation was induced by phenobarbital, whereas the formation of non-K-region metabolites increased after BkF treatment in the case of benz[c]acridine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
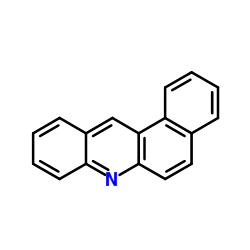 |
benz(a)acridine
CAS:225-11-6 |
C17H11N |
|
Substituted benz[a]acridines and benz[c]acridines as mammali...
2000-05-01 [Bioorg. Med. Chem. 8(5) , 1171-82, (2000)] |
|
Benz[a]acridine.
1983-12-01 [IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 32 , 123-7, (1983)] |
|
Mutagenicity of diol-epoxides and tetrahydroepoxides of benz...
1983-04-01 [Cancer Res. 43(4) , 1656-62, (1983)] |
|
Experimental studies on the carcinogenicity of five nitrogen...
1983-08-01 [Cancer Lett. 20(1) , 97-101, (1983)] |
|
The accumulation and disposition of benz(a)acridine in the f...
1981-09-01 [Arch. Environ. Contam. Toxicol. 10(5) , 561-9, (1981)] |