benz(a)acridine
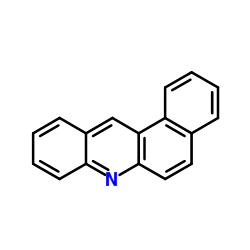
benz(a)acridine structure
|
Common Name | benz(a)acridine | ||
|---|---|---|---|---|
| CAS Number | 225-11-6 | Molecular Weight | 229.276 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 446.2±14.0 °C at 760 mmHg | |
| Molecular Formula | C17H11N | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 201.4±12.7 °C | |
|
Substituted benz[a]acridines and benz[c]acridines as mammalian topoisomerase poisons.
Bioorg. Med. Chem. 8(5) , 1171-82, (2000) Coralyne and several other synthetic benzo[a,g]quinolizium derivatives related to protoberberine alkaloids have exhibited activity as topoisomerase poisons. These compounds are characterized by the presence of a positively charged iminium group, which has bee... |
|
|
Benz[a]acridine.
IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 32 , 123-7, (1983)
|
|
|
Mutagenicity of diol-epoxides and tetrahydroepoxides of benz(a)acridine and benz(c)acridine in bacteria and in mammalian cells.
Cancer Res. 43(4) , 1656-62, (1983)
|
|
|
On the metabolic activation of benz[a]acridine and benz[c]acridine by rat liver and lung microsomes.
Cancer Lett. 16(3) , 297-306, (1982) The metabolism of benz[a]- and benz[c]acridine by liver and lung microsomes from untreated, phenobarbital (PB)-treated and benzo[k]fluoranthene (BkF)-treated rats has been studied by gas chromatography/mass spectrometry (GC/MS). Epoxidation and hydrolysis of ... |
|
|
Experimental studies on the carcinogenicity of five nitrogen containing polycyclic aromatic compounds directly injected into rat lungs.
Cancer Lett. 20(1) , 97-101, (1983) Using a beeswax/tricaprylin mixture as vehicle, three doses each of acridine, benz[a]acridine (BaAC), benz[c]acridine (BcAC), dibenz[a,h]-acridine (DBa,hAC) and dibenz[a,j]acridine (DBa,jAC) were injected into the lungs of 35 female Osborne-Mendel rats per gr... |
|
|
The accumulation and disposition of benz(a)acridine in the fathead minnow, Pimephales promelas.
Arch. Environ. Contam. Toxicol. 10(5) , 561-9, (1981) The bioconcentration and metabolism of benz(a)acridine in fathead minnows (Pimephales promelas) was investigated using 14C-labelled benz(a)acridine. The rates of uptake, elimination, and metabolic transformation of benz(a)acridine were estimated in the fish. ... |
|
|
Induction of the rat hepatic microsomal mixed-function oxidases by two aza-arenes. A comparison with their non-heterocyclic analogues.
Biochem. Pharmacol. 37(23) , 4565-71, (1988) The ability of the aza-aromatic polycyclic aromatic hydrocarbons 10-azobenz(a)pyrene and benz(a)acridine to induce the rat hepatic microsomal mixed-function oxidases was compared to that of their non-heterocyclic analogues benz(a)pyrene and benz(a)anthracene ... |
|
|
Heteroatom effects in chemical carcinogenesis: effects of ring heteroatoms on ease of carbocation formation.
Cancer Biochem. Biophys. 7(1) , 53-60, (1983) The presence of a heteroatom can influence the ease with which a PAH diol-epoxide forms a triol carbocation. The influence of the heteroatom should be greatest when it is located where the PAH undergoes maximum charge change upon ionization. Extended Hückel, ... |
|
|
Differentiated genotoxic response of carcinogenic and non-carcinogenic benzacridines and metabolites in rat hepatoma cells.
Carcinogenesis 6(3) , 455-7, (1985) Two closely related hepatoma cell lines were examined for their genotoxic response to benacridines and their metabolites by the appearance of alkaline labile DNA sites: H5, a dedifferentiated line expressing cytochrome P-448-dependent mono-oxygenase(s); and H... |
|
|
Diverse biological activities displayed by phenothiazines, benzo[a]phenothiazines and benz[c]acridins (review).
Anticancer Res. 13(4) , 1019-25, (1993) This review summarizes our experiments which are investigating the relationship between the structure and activity of mainly phenothiazines, benzo[a]phenothiazines and benz[c]acridines. Phenothiazines had potent antiplasmid and antibacterial activities, but i... |