| Structure | Name/CAS No. | Articles |
|---|---|---|
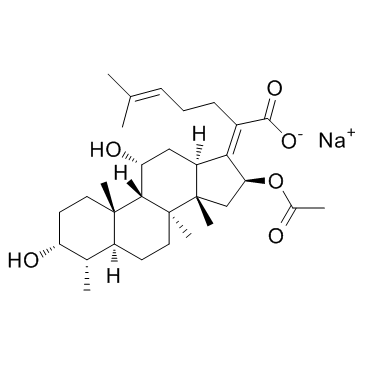 |
Fusidic acid (sodium salt)
CAS:751-94-0 |
|
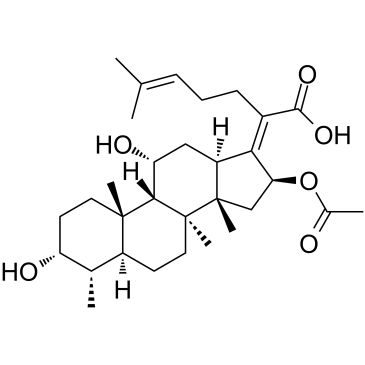 |
fusidic acid
CAS:6990-06-3 |
|
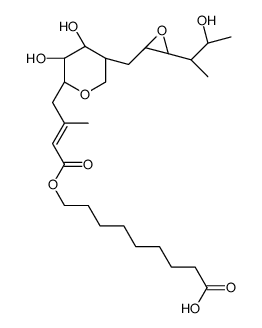 |
Lithium mupirocin
CAS:73346-79-9 |
|
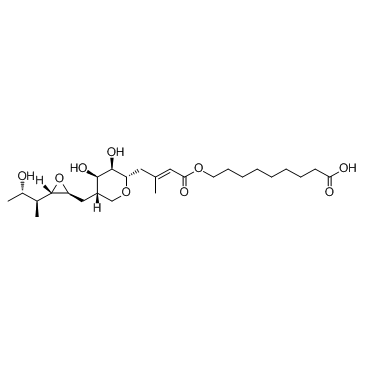 |
Mupirocin
CAS:12650-69-0 |