Novel approach to 1,5-benzodiazepine-2-ones containing peptoid backbone via one-pot diketene-based Ugi-4CR.
Nasrin Zohreh, Abdolali Alizadeh, Hamid Reza Bijanzadeh, Log-Guan Zhu
Index: J. Comb. Chem. 12(4) , 497-502, (2010)
Full Text: HTML
Abstract
An efficient and simple route for preparation of substituted 1,5-benzodiazepine-2-one containing peptoid backbone is presented. The classical Ugi reaction is considerably extended by application of o-phenylenediamine and diketene as amine and oxo component. 1,3-Dihydro-1,5-benzodiazepine-2-one is generated in situ from these two building blocks combined with isocyanide and aromatic or aliphatic carboxylic acid to assemble the multifunctionalized titled scaffold in high yields. The reaction is performed in the mixture of toluene/CH(2)Cl(2) under reflux condition without catalyst. Conformational isomerism is seen in the solution phase because of restricted free rotation around amide and C-CO bands due to steric bulk of substitutions. In single crystal state, the product is found to possess dimeric structure arising from intermolecular hydrogen bonding.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
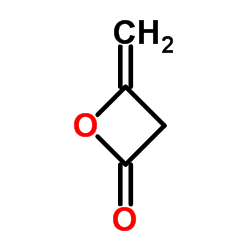 |
Diketene
CAS:674-82-8 |
C4H4O2 |
|
Chemical reactivity and biological activity of diketene.
2008-10-01 [Chem. Res. Toxicol. 21(10) , 1964-9, (2008)] |
|
One-pot multicomponent synthesis of 1-aryl-5-methyl-N-R2-1H-...
2009-01-01 [J. Comb. Chem. 11(3) , 481-5, (2009)] |
|
Interactions between earthworm hemolysins and sheep red bloo...
1989-08-07 [Biochim. Biophys. Acta 983(2) , 193-8, (1989)] |
|
New one-pot four-component synthesis of disubstituted pyrido...
2009-01-01 [J. Comb. Chem. 11(3) , 375-7, (2009)] |
|
Bisaryldiketene derivatives: A new class of selective ligand...
2010-12-01 [Bioorg. Med. Chem. 18(23) , 8235-42, (2010)] |