Diketene
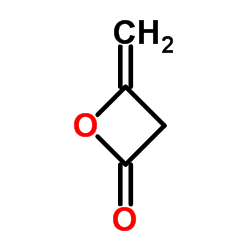
Diketene structure
|
Common Name | Diketene | ||
|---|---|---|---|---|
| CAS Number | 674-82-8 | Molecular Weight | 84.073 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 127.4±0.0 °C at 760 mmHg | |
| Molecular Formula | C4H4O2 | Melting Point | -7.5°C | |
| MSDS | Chinese USA | Flash Point | 34.4±0.0 °C | |
| Symbol |


GHS02, GHS06 |
Signal Word | Danger | |
|
Chemical reactivity and biological activity of diketene.
Chem. Res. Toxicol. 21(10) , 1964-9, (2008) The alkylating potential of diketene (4-methylene-2-oxetanone), the basic unit of many derivatives of pesticides, chemicals, pharmaceuticals, and dyestuffs, was investigated kinetically. The nucleophile 4-( p-nitrobenzyl)pyridine (NBP), a trap for alkylating ... |
|
|
Novel approach to 1,5-benzodiazepine-2-ones containing peptoid backbone via one-pot diketene-based Ugi-4CR.
J. Comb. Chem. 12(4) , 497-502, (2010) An efficient and simple route for preparation of substituted 1,5-benzodiazepine-2-one containing peptoid backbone is presented. The classical Ugi reaction is considerably extended by application of o-phenylenediamine and diketene as amine and oxo component. 1... |
|
|
One-pot multicomponent synthesis of 1-aryl-5-methyl-N-R2-1H-1,2,3-triazole-4-carboxamides: an easy procedure for combinatorial chemistry.
J. Comb. Chem. 11(3) , 481-5, (2009) A convenient synthetic protocol was elaborated for creation of combinatorial libraries of 1-(R(1)-phenyl)-5-methyl-N-R(2)-1H-1,2,3-triazole-4-carboxamides. As starting materials, commercially available or readily prepared azides, amines, and diketene were sel... |
|
|
Interactions between earthworm hemolysins and sheep red blood cell membranes.
Biochim. Biophys. Acta 983(2) , 193-8, (1989) The hemolytic activity exhibited by the coelomic fluid of the Annelid Eisenia fetida andrei is mediated by two lipoproteins of mass 40 and 45 kDa, each of them capable of hemolysis. Such an activity is not inhibited by zymosan, inulin or lipopolysaccharide (L... |
|
|
New one-pot four-component synthesis of disubstituted pyrido[2,3-d]pyrimidine-6-carboxamide derivatives.
J. Comb. Chem. 11(3) , 375-7, (2009) In this work, 1,2,3,4,5,8-hexahydro-1,3,7-trimethyl-2,4-dioxopyrido[2,3-d]pyrimidine-6-carboxamide derivatives were synthesized in a simple and efficient method from the four-component condensation reaction of diketene, an aliphatic or aromatic amine, an arom... |
|
|
Bisaryldiketene derivatives: A new class of selective ligands for c-myc G-quadruplex DNA.
Bioorg. Med. Chem. 18(23) , 8235-42, (2010) A series of bisaryldiketene derivatives were designed and synthesized as a new class of specific G-quadruplex ligands. The ligand-quadruplex interactions were further evaluated by FRET, ITC, and PCR stop assay. In contrast to most of the G-quadruplex ligands ... |
|
|
UV-Induced unimolecular photochemistry of diketene isolated in cryogenic inert matrices.
J. Phys. Chem. A 116(9) , 2131-40, (2012) Diketene (C(4)H(4)O(2)) monomers were isolated in cryogenic Ar (15 K) and Xe (30 K) matrices. The infrared (IR) spectra of the freshly deposited matrices show that diketene monomers exclusively adopt the 4-methylene-oxetan-2-one form. In situ photochemical tr... |
|
|
Effects of amino group modification in discoidal apolipoprotein A-I-egg phosphatidylcholine-cholesterol complexes on their reactions with lecithin:cholesterol acyltransferase.
Biochemistry 24(14) , 3508-13, (1985) Discoidal complexes of human apolipoprotein A-I-egg phosphatidylcholine-cholesterol were prepared by the sodium cholate dialysis procedure and were reacted to varying extents with the amino group reagents citraconic anhydride, diketene, and formaldehyde in th... |
|
|
Functional residues at the active site of horse liver phosphopantothenoylcysteine decarboxylase.
FEBS Lett. 231(1) , 192-6, (1988) Horse liver phosphopantothenoylcysteine decarboxylase (EC 4.1.1.36) is rapidly inactivated by N-acetoacetylation with diketene following a pseudo-first-order kinetics: the presence of substrate quantitatively protects against this inactivation. Histidine phot... |
|
|
Identification of essential amino acid residues in clostridium histolyticum collagenase using chemical modification reactions.
Biochem. Biophys. Res. Commun. 102(1) , 243-9, (1981)
|