Bisaryldiketene derivatives: A new class of selective ligands for c-myc G-quadruplex DNA.
Dan Peng, Jia-Heng Tan, Shuo-Bin Chen, Tian-Miao Ou, Lian-Quan Gu, Zhi-Shu Huang
Index: Bioorg. Med. Chem. 18(23) , 8235-42, (2010)
Full Text: HTML
Abstract
A series of bisaryldiketene derivatives were designed and synthesized as a new class of specific G-quadruplex ligands. The ligand-quadruplex interactions were further evaluated by FRET, ITC, and PCR stop assay. In contrast to most of the G-quadruplex ligands reported so far, which comprise an extended aromatic ring, these compounds are neither polycyclic nor macrocyclic, but have a non-aromatic and relative flexible linker between two quinoline moieties enabling the conformation of compounds to be flexible. Our results showed that these bisaryldiketene derivatives could selectively recognize G-quadruplex DNA rather than binding to duplex DNA. Moreover, they showed promising discrimination between different G-quadruplex DNA. The primary binding affinity of ligand M2 for c-myc G-quadruplex DNA was over 200 times larger than that for telomere G-quadruplex DNA.Copyright © 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
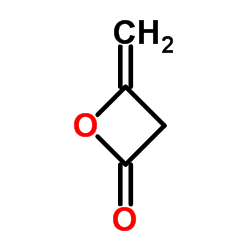 |
Diketene
CAS:674-82-8 |
C4H4O2 |
|
Chemical reactivity and biological activity of diketene.
2008-10-01 [Chem. Res. Toxicol. 21(10) , 1964-9, (2008)] |
|
Novel approach to 1,5-benzodiazepine-2-ones containing pepto...
2010-07-12 [J. Comb. Chem. 12(4) , 497-502, (2010)] |
|
One-pot multicomponent synthesis of 1-aryl-5-methyl-N-R2-1H-...
2009-01-01 [J. Comb. Chem. 11(3) , 481-5, (2009)] |
|
Interactions between earthworm hemolysins and sheep red bloo...
1989-08-07 [Biochim. Biophys. Acta 983(2) , 193-8, (1989)] |
|
New one-pot four-component synthesis of disubstituted pyrido...
2009-01-01 [J. Comb. Chem. 11(3) , 375-7, (2009)] |