| Structure | Name/CAS No. | Articles |
|---|---|---|
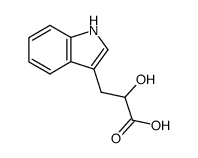 |
DL-INDOLE-3-LACTIC ACID
CAS:832-97-3 |
|
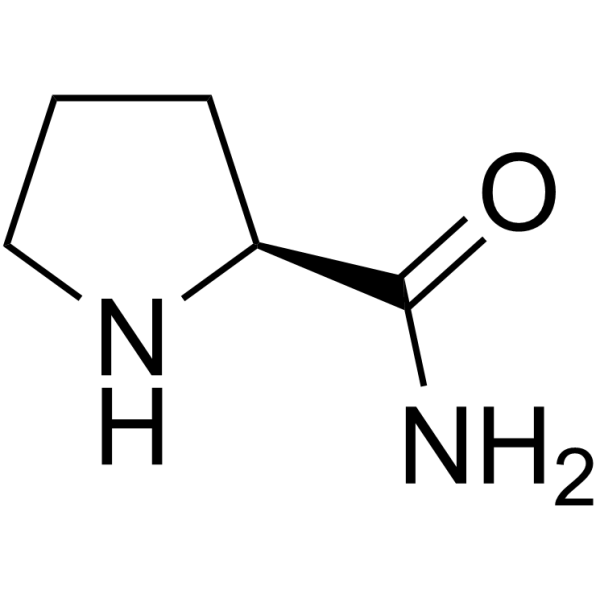 |
L-Prolinamide
CAS:7531-52-4 |