L-Prolinamide
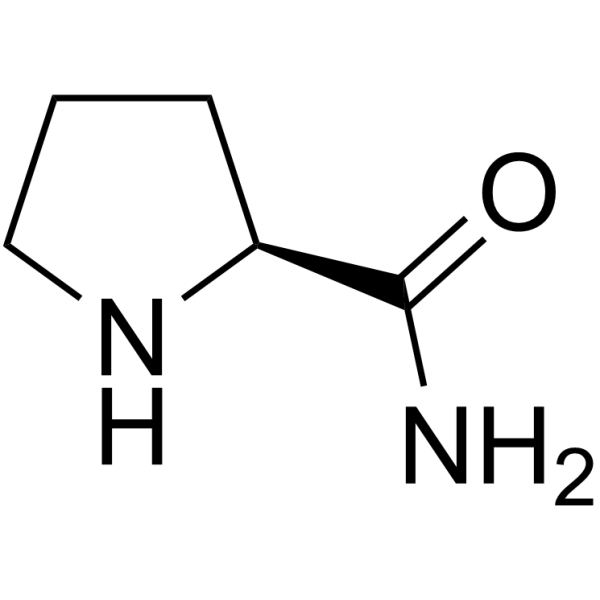
L-Prolinamide structure
|
Common Name | L-Prolinamide | ||
|---|---|---|---|---|
| CAS Number | 7531-52-4 | Molecular Weight | 114.146 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 303.6±31.0 °C at 760 mmHg | |
| Molecular Formula | C5H10N2O | Melting Point | 95-97 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 137.4±24.8 °C | |
|
Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor.
Nat. Chem. Biol. 5 , 45-52, (2009) Transporter-related nutrient sensors, called transceptors, mediate nutrient activation of signaling pathways through the plasma membrane. The mechanism of action of transporting and nontransporting transceptors is unknown. We have screened 319 amino acid anal... |
|
|
Enantioseparation of amino acids and alpha-hydroxy acids on ligand-exchange continuous beds by capillary electrochromatography.
Electrophoresis 31 , 1517-1520, (2010) A new chiral stationary phase based on continuous bed (CB) technology using L-prolinamide as a chiral selector was prepared. Its ability for enantioseparation of amino acids and alpha-hydroxy acids by ligand-exchange CEC was compared with that of a CB contain... |
|
|
d-Glucosamine in a chimeric prolinamide organocatalyst for direct asymmetric aldol addition
Carbohydr. Res. 356 , 273-7, (2012) O-TBDPS D-glucosamine coupled with l-proline is reported to act as an efficient organocatalyst in the accomplishment of direct aldol reactions. Excellent results, in terms of chemical yields, as well as diastereomeric and enantiomeric ratios, are reported for... |
|
|
The mimic of type II aldolases chemistry: asymmetric synthesis of beta-hydroxy ketones by direct aldol reaction.
Chem. Biol. Drug Des. 76(2) , 181-6, (2010) An efficient direct aldol reaction has been developed for the synthesis of chiral beta-hydroxy ketone using a combination of C(1)-symmetric chiral prolinamides based on o-phenylenediamine and zinc triflate as catalyst. The reaction was convenient to carry out... |
|
|
Crystal structures and spectroscopic properties of ester amide and diamide of squaric acid with prolinamide
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 72(3) , 502-9, (2009) We report the synthesis, spectroscopic and structural elucidation of two prolinamide derivatives of squaric acid, i.e. prolinamide ester amide of squaric acid ethyl ester ( 1) and prolinamide diamide of squaric acid dihydrate ( 2). Both compounds crystallize ... |
|
|
Chiral separation of NBD-amino acids by ligand-exchange micro-channel electrophoresis.
Anal. Sci. 21 , 67-71, (2005) The chiral separation of amino acid derivatives by ligand-exchange electrophoresis in a microchannel chip was performed for the first time. A Cu(II) complex with L-prolinamide was used as a chiral selector. The migration behaviors of eleven NBD-DL-amino acids... |
|
|
Chemically L-prolinamide-modified monolithic silica column for enantiomeric separation of dansyl amino acids and hydroxy acids by capillary electrochromatography and mu-high performance liquid chromatography.
Electrophoresis 22(15) , 3339-46, (2001) A silica-based chiral monolithic column prepared by sol-gel process and chemical modification of chiral selector was used for enantioseparation of dansyl amino acids and hydroxy acids by capillary electrochromatography (CEC) and mu-high-performance liquid chr... |
|
|
Solid-state-trapped reactive ammonium carbamate self-derivative salts of prolinamide.
ChemistryOpen 2 , 194-9, (2014) Single crystals for two polymorphs of the ammonium carbamate self-derivative salt of prolinamide have been successfully obtained and characterized. Decarbonation of the carbamate salts was monitored by calorimetry, confirming stabilization of the reactive car... |
|
|
A tripeptide-like prolinamide-thiourea as an aldol reaction catalyst.
Org. Biomol. Chem. 10(29) , 5613-9, (2012) A tripeptide-like prolinamide-thiourea catalyst with (S)-proline, (1S,2S)-diphenylethylenediamine and (S)-di-tert-butyl aspartate as building blocks provides the products of the reaction between ketones and aromatic aldehydes in high to quantitative yields an... |
|
|
HCV NS5A replication complex inhibitors. Part 2: investigation of stilbene prolinamides.
Bioorg. Med. Chem. Lett. 22(19) , 6063-6, (2012) In a previous disclosure,(1) we reported the dimerization of an iminothiazolidinone to form 1, a contributor to the observed inhibition of HCV genotype 1b replicon activity. The dimer was isolated via bioassay-guided fractionation experiments and shown to be ... |