| Structure | Name/CAS No. | Articles |
|---|---|---|
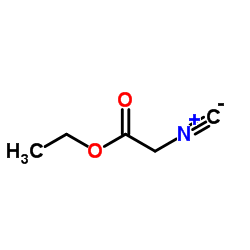 |
Ethyl isocyanoacetate
CAS:2999-46-4 |
Chikashi Kanazawa, Shin Kamijo, Yoshinori Yamamoto
Index: J. Am. Chem. Soc. 128 , 10662, (2006)
Full Text: HTML
The copper-catalyzed reaction between two different isocyanides produces the corresponding heteroaromatization products, imidazoles, in good yields. The reaction proceeds most probably through the activation of the sp3 C-H bond in the isocyanides by a copper catalyst, followed by a [3 + 2] cycloaddition between the cuprioisocyanide intermediates and arylisocyanides.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Ethyl isocyanoacetate
CAS:2999-46-4 |
C5H7NO2 |
|
Fully automated continuous flow synthesis of 4,5-disubstitut...
2006-11-09 [Org. Lett. 8 , 5231, (2006)] |
|
Synthesis and evaluation of imidazo[1,5-a][1,4]benzodiazepin...
1993-04-16 [J. Med. Chem. 36 , 1001, (1993)] |
|
Double nucleophilic attack on isocyanide carbon: a synthetic...
2012-12-28 [Chem. Commun. (Camb.) 48(100) , 12228-30, (2012)] |
|
[Tetrahedron Lett. 35 , 2493, (1994)] |
|
[Tetrahedron Lett. 34 , 5463, (1993)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved