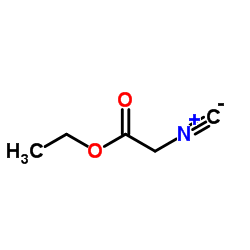
Ethyl isocyanoacetate
Ethyl isocyanoacetate structure
|
Common Name | Ethyl isocyanoacetate | ||
|---|---|---|---|---|
| CAS Number | 2999-46-4 | Molecular Weight | 113.115 | |
| Density | 1.035 g/mL at 25 °C(lit.) | Boiling Point | 194-196 °C(lit.) | |
| Molecular Formula | C5H7NO2 | Melting Point | 194-196ºC | |
| MSDS | Chinese USA | Flash Point | 184 °F | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Synthesis of imidazoles through the copper-catalyzed cross-cycloaddition between two different isocyanides.
J. Am. Chem. Soc. 128 , 10662, (2006) The copper-catalyzed reaction between two different isocyanides produces the corresponding heteroaromatization products, imidazoles, in good yields. The reaction proceeds most probably through the activation of the sp3 C-H bond in the isocyanides by a copper ... |
|
|
Fully automated continuous flow synthesis of 4,5-disubstituted oxazoles.
Org. Lett. 8 , 5231, (2006) [Structure: see text] A multipurpose mesofluidic flow reactor capable of producing gram quantities of material has been developed as an automated synthesis platform for the rapid on-demand synthesis of key building blocks and small exploratory libraries. The ... |
|
|
Synthesis and evaluation of imidazo[1,5-a][1,4]benzodiazepine esters with high affinities and selectivities at "diazepam-insensitive" benzodiazepine receptors.
J. Med. Chem. 36 , 1001, (1993) A series of imidazo[1,5-a][1,4]benzodiazepine esters have been synthesized with varying ester side chains and 8-position substituents. The affinities of these compounds were evaluated at both "diazepam-insensitive" (DI) and diazepam-sensitive (DS) subtypes of... |
|
|
Double nucleophilic attack on isocyanide carbon: a synthetic strategy for 7-aza-tetrahydroindoles.
Chem. Commun. (Camb.) 48(100) , 12228-30, (2012) A novel and efficient route for the synthesis of 7-aza-tetrahydroindoles from N-aryl/alkyl-alkenoylacetamides and ethyl isocyanoacetate is described. A mechanism, involving a stepwise [3+2] cycloaddition-intramolecular aza-Michael addition cascade, is propose... |
|
|
Tetrahedron Lett. 35 , 2493, (1994)
|
|
|
Tetrahedron Lett. 34 , 5463, (1993)
|
|
|
Tetrahedron Lett. 47 , 5481, (2006)
|