Extraordinary hyperconjugation of the methyl group in the S(1) state of 8-methylquinoline.
R Yang, S G Schulman
Index: Luminescence 16(2) , 129-33, (2001)
Full Text: HTML
Abstract
8-Methylquinoline is unique among the monomethylquinolines in the red-shift it shows in the absorption band derived from the short axis polarized ((1)L(a) <-- (1)A) electronic transition, relative to that in quinoline itself. The effect is even more pronounced in the 8-methylquinolinium cation, with the (1)L(a) band clearly emerging on the long-wavelength side of the (1)L(b) band, which is normally the longest wavelength absorption band in quinolines substituted with weakly interacting functional groups. The fluorescences of 8-methylquinoline and its conjugate acid show a pH dependence which is typical of N-heterocylic bases that undergo hydrolysis in the lowest excited singlet state. These observations are explained in terms of hyperconjugation in the lowest excited singlet state, with electronic charge being swept into the quinoline ring by coupling of the short-axis polarized transition of the ring system with the electrons of the methyl group in the 8-position of the ring.Copyright 2001 John Wiley & Sons, Ltd.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
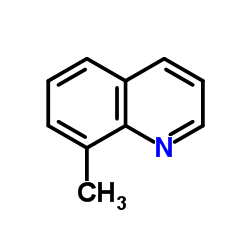 |
8-Methylquinoline
CAS:611-32-5 |
C10H9N |
|
A P450 BM-3 mutant hydroxylates alkanes, cycloalkanes, arene...
2001-06-15 [J. Biotechnol. 88(2) , 167-71, (2001)] |
|
The hepatic metabolism of two methylquinolines.
1993-05-01 [Carcinogenesis 14(5) , 1041-7, (1993)] |
|
Carcinogenicity of quinoline, 4- and 8-methylquinoline and b...
1988-07-01 [Food Chem. Toxicol. 26(7) , 625-9, (1988)] |
|
Synthetic routes to N-heterocyclic carbene complexes: pyridi...
2007-01-01 [Angew. Chem. Int. Ed. Engl. 46(19) , 3405-8, (2007)] |
|
Pd-catalyzed C-H fluorination with nucleophilic fluoride.
2012-08-17 [Org. Lett. 14(16) , 4094-7, (2012)] |