8-Methylquinoline
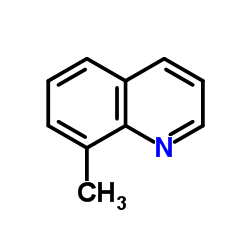
8-Methylquinoline structure
|
Common Name | 8-Methylquinoline | ||
|---|---|---|---|---|
| CAS Number | 611-32-5 | Molecular Weight | 143.185 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 247.5±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H9N | Melting Point | -80 °C | |
| MSDS | Chinese USA | Flash Point | 105.0±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
A P450 BM-3 mutant hydroxylates alkanes, cycloalkanes, arenes and heteroarenes.
J. Biotechnol. 88(2) , 167-71, (2001) P450 monooxygenases from microorganisms, similar to those of eukaryotic mitochondria, display a rather narrow substrate specificity. For native P450 BM-3, no other substrates than fatty acids or an indolyl-fatty acid derivative have been reported (Li, Q.S., S... |
|
|
The hepatic metabolism of two methylquinolines.
Carcinogenesis 14(5) , 1041-7, (1993) The hepatic microsomal metabolism of the carcinogenic 8-methylquinoline (8MQ) and its noncarcinogenic isomer, 6-methylquinoline (6MQ), were compared for preparations from control rats and rats pretreated with phenobarbital or 3-methylcholanthrene. For each co... |
|
|
Carcinogenicity of quinoline, 4- and 8-methylquinoline and benzoquinolines in newborn mice and rats.
Food Chem. Toxicol. 26(7) , 625-9, (1988) The relative tumorigenic activity of quinoline, 4-methylquinoline, 8-methylquinoline, and all three isomeric benzoquinolines was evaluated in newborn CD-1 mice and Sprague-Dawley rats. In the newborn-mouse bioassay, 0.25, 0.5 and 1.0 mumol of each compound in... |
|
|
Synthetic routes to N-heterocyclic carbene complexes: pyridine-carbene tautomerizations.
Angew. Chem. Int. Ed. Engl. 46(19) , 3405-8, (2007)
|
|
|
Pd-catalyzed C-H fluorination with nucleophilic fluoride.
Org. Lett. 14(16) , 4094-7, (2012) The palladium-catalyzed C-H fluorination of 8-methylquinoline derivatives with nucleophilic fluoride is reported. This transformation involves the use of AgF as the fluoride source in combination with a hypervalent iodine oxidant. Both the scope and mechanism... |
|
|
Palladium-catalyzed fluorination of carbon-hydrogen bonds.
J. Am. Chem. Soc. 128(22) , 7134-5, (2006) This communication describes the development of a new Pd-catalyzed method for the fluorination of carbon-hydrogen bonds. A key step of these transformations involves palladium-mediated carbon-fluorine coupling-a much sought after, but previously unprecedented... |
|
|
Extraordinary hyperconjugation of the methyl group in the S(1) state of 8-methylquinoline.
Luminescence 16(2) , 129-33, (2001) 8-Methylquinoline is unique among the monomethylquinolines in the red-shift it shows in the absorption band derived from the short axis polarized ((1)L(a) <-- (1)A) electronic transition, relative to that in quinoline itself. The effect is even more pronounce... |