Kinetic resolution of racemic 2-hydroxy-γ-butyrolactones by asymmetric esterification using diphenylacetic acid with pivalic anhydride and a chiral acyl-transfer catalyst.
Kenya Nakata, Kouya Gotoh, Keisuke Ono, Kengo Futami, Isamu Shiina
Index: Org. Lett. 15(6) , 1170-3, (2013)
Full Text: HTML
Abstract
Various optically active 2-hydroxy-γ-butyrolactone derivatives are produced via the kinetic resolution of racemic 2-hydroxy-γ-butyrolactones with diphenylacetic acid using pivalic anhydride and (R)-benzotetramisole ((R)-BTM), a chiral acyl-transfer catalyst. Importantly, the substrate scope of this novel protocol is fairly broad (12 examples, s-value; up to over 1000). In addition, we succeeded in disclosing the reaction mechanism to afford high enantioselectivity using theoretical calculations and expounded on the substituent effects at the C-3 positions in 2-hydroxylactones.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
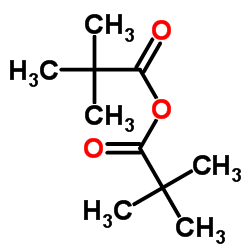 |
TRIMETHYLACETIC ANHYDRIDE
CAS:1538-75-6 |
C10H18O3 |
|
The concept of superactive esters. Could peptide synthesis b...
1994-03-01 [Int. J. Pept. Protein Res. 43 , 312, (1994)] |
|
Observation and elimination of N-acetylation of oligonucleot...
2001-05-07 [Bioorg. Med. Chem. Lett. 11(9) , 1105-1107, (2001)] |
|
New synthetic substrates of mammalian nucleotide excision re...
2013-07-01 [Nucleic Acids Res. 41 , e123, (2013)] |
|
Structural Basis for Substrate Specificity in Adenosylcobala...
2015-11-06 [J. Biol. Chem. 290 , 26882-98, (2015)] |
|
Kinetic resolution of the racemic 2-hydroxyalkanoates using ...
2010-01-04 [Chemistry 16(1) , 167-72, (2010)] |