TRIMETHYLACETIC ANHYDRIDE
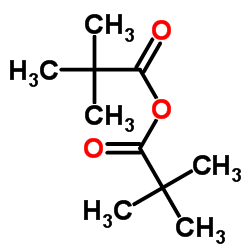
TRIMETHYLACETIC ANHYDRIDE structure
|
Common Name | TRIMETHYLACETIC ANHYDRIDE | ||
|---|---|---|---|---|
| CAS Number | 1538-75-6 | Molecular Weight | 186.248 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 193.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H18O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 57.2±0.0 °C | |
| Symbol |


GHS02, GHS05 |
Signal Word | Danger | |
|
Kinetic resolution of racemic 2-hydroxy-γ-butyrolactones by asymmetric esterification using diphenylacetic acid with pivalic anhydride and a chiral acyl-transfer catalyst.
Org. Lett. 15(6) , 1170-3, (2013) Various optically active 2-hydroxy-γ-butyrolactone derivatives are produced via the kinetic resolution of racemic 2-hydroxy-γ-butyrolactones with diphenylacetic acid using pivalic anhydride and (R)-benzotetramisole ((R)-BTM), a chiral acyl-transfer catalyst. ... |
|
|
The concept of superactive esters. Could peptide synthesis be improved by inventing superactive esters?
Int. J. Pept. Protein Res. 43 , 312, (1994) According to the concept presented, esters forming an amide (peptide) bond by the mechanism SN#DN or SN#*DN involving fast decay of the tetrahedral intermediate may behave as 'superactive acylating reagents'. These should render coupling involving less reacti... |
|
|
Observation and elimination of N-acetylation of oligonucleotides prepared using fast-deprotecting phosphoramidites and ultra-mild deprotection.
Bioorg. Med. Chem. Lett. 11(9) , 1105-1107, (2001) Commercially available 'fast-deprotecting' phosphoramidites are useful for synthesizing oligonucleotides containing alkali-sensitive nucleotides. However, N-acetylated oligonucleotides were observed during solid-phase synthesis using 'fast-deprotecting' phosp... |
|
|
New synthetic substrates of mammalian nucleotide excision repair system.
Nucleic Acids Res. 41 , e123, (2013) DNA probes for the studies of damaged strand excision during the nucleotide excision repair (NER) have been designed using the novel non-nucleosidic phosphoramidite reagents that contain N-[6-(9-antracenylcarbamoyl)hexanoyl]-3-amino-1,2-propandiol (nAnt) and ... |
|
|
Structural Basis for Substrate Specificity in Adenosylcobalamin-dependent Isobutyryl-CoA Mutase and Related Acyl-CoA Mutases.
J. Biol. Chem. 290 , 26882-98, (2015) Acyl-CoA mutases are a growing class of adenosylcobalamin-dependent radical enzymes that perform challenging carbon skeleton rearrangements in primary and secondary metabolism. Members of this class of enzymes must precisely control substrate positioning to p... |
|
|
Kinetic resolution of the racemic 2-hydroxyalkanoates using the enantioselective mixed-anhydride method with pivalic anhydride and a chiral acyl-transfer catalyst.
Chemistry 16(1) , 167-72, (2010) A variety of optically active 2-hydroxyalkanoates and the corresponding 2-acyloxyalkanoates are produced by the kinetic resolution of racemic 2-hydroxyalkanoates by using achiral 2,2-diarylacetic acid with hindered carboxylic anhydrides as the coupling reagen... |
|
|
Single-step, quantitative derivatization of amino, carboxyl, and hydroxyl groups in iodothyronine amino acids with ethanolic pivalic anhydride containing 4-dimethylaminopyridine.
Anal. Biochem. 153(1) , 159-65, (1986) Reaction of thyroxine with ethanol and pivalic anhydride in the presence of 4-dimethylaminopyridine quantitatively forms N,O-dipivalyl thyroxine ethyl ester. Other iodothyronines react similarly and the procedure is moisture insensitive. Apparently this react... |
|
|
Bull. Chem. Soc. Jpn. 67 , 210, (1994)
|
|
|
Pharmacol. Res. 10 , 68, (1993)
|
|
|
Aust. J. Chem. 60 , 75, (2007)
|