| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
H-Phg-OH
CAS:2935-35-5 |
|
 |
2-Anilinoethanol
CAS:122-98-5 |
|
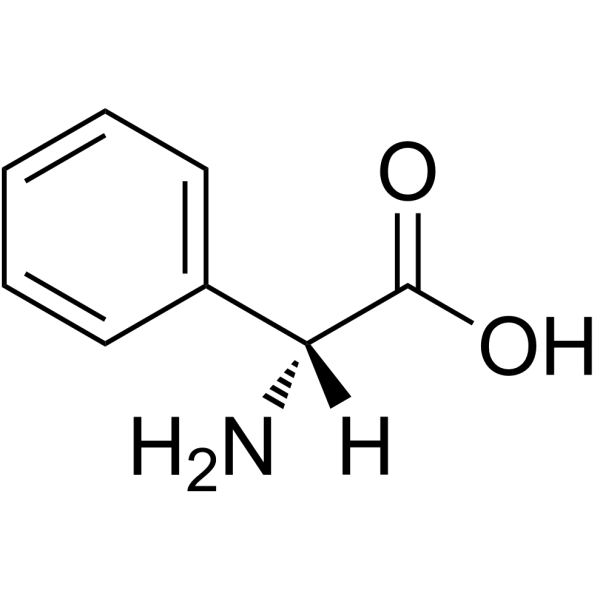 |
D-2-Phenylglycine
CAS:875-74-1 |
|
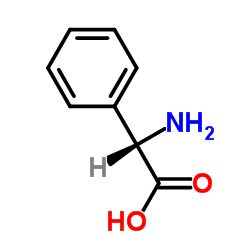 |
2-Phenylglycine
CAS:2835-06-5 |