Journal of chromatography. B, Biomedical sciences and applications
1997-12-19
Biotransformation of 17-alkyl steroids in the equine: high-performance liquid chromatography-mass spectrometric and gas chromatography-mass spectrometric analysis of fluoxymesterone metabolites in urine samples.
S M Stanley, S Kent, J P Rodgers
Index: J. Chromatogr. B. Biomed. Sci. Appl. 704 , 119, (1997)
Full Text: HTML
Abstract
In this study the equine metabolism of fluoxymesterone (9alpha-fluoro-11beta-17beta-dihydroxy-17alpha-meth ylandrost-4-ene-3-one) given orally has been investigated. The parent material was not detected, but two major 16-hydroxy metabolites which corresponded to a mono- and a di-hydroxylation product were evident. One of the hydroxylation positions was identified as C-16. Phase II metabolism in the form of glucuronide formation was also common. These steroids will provide target compounds for confirming abuse of this drug in the horse.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
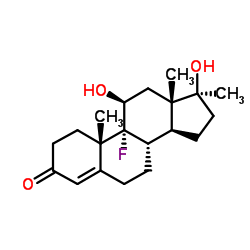 |
Fluoxymesterone
CAS:76-43-7 |
C20H29FO3 |
Related Articles:
More...
|
Rat liver lysosomal and mitochondrial activities are modifie...
1999-02-01 [Med. Sci Sports Exerc. 31 , 243, (1999)] |
|
Comparison of chemotherapy with chemohormonal therapy as fir...
2000-01-01 [J. Clin. Oncol. 18(2) , 262-6, (2000)] |
|
Sample size calculations for the two-sample problem using th...
2001-02-28 [Stat. Med. 20(4) , 557-79, (2001)] |
|
Toxic effects of anabolic-androgenic steroids in primary rat...
1995-08-01 [J. Pharmacol. Toxicol. Methods 33(4) , 187-95, (1995)] |
|
Randomized trial of tamoxifen alone or combined with fluoxym...
2006-07-01 [Breast Cancer Res. Treat. 98(2) , 217-22, (2006)] |