Sample size calculations for the two-sample problem using the multiplicative intensity model.
M V Bernardo, D P Harrington
Index: Stat. Med. 20(4) , 557-79, (2001)
Full Text: HTML
Abstract
In this paper we propose formulae for calculating the expected number of events or, alternatively, the required trial duration, for clinical trials involving two treatment groups in which patients may potentially experience multiple events and the data will be analysed using a multiplicative intensity (MI) model. We use a partial likelihood-based approach and examine in detail two MI models: one that includes a binary treatment variable as the only covariate and a three-state Markov process model in which a binary time-varying covariate is added to the previous model. For the simpler model, our formula coincides with those derived by Cook using full likelihood methods. We present applications of the derived formulae to chronic granulomatous disease and breast cancer data sets.Copyright 2001 John Wiley & Sons, Ltd.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
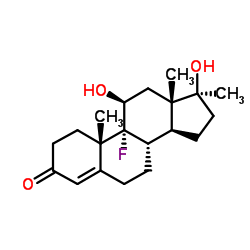 |
Fluoxymesterone
CAS:76-43-7 |
C20H29FO3 |
|
Rat liver lysosomal and mitochondrial activities are modifie...
1999-02-01 [Med. Sci Sports Exerc. 31 , 243, (1999)] |
|
Biotransformation of 17-alkyl steroids in the equine: high-p...
1997-12-19 [J. Chromatogr. B. Biomed. Sci. Appl. 704 , 119, (1997)] |
|
Comparison of chemotherapy with chemohormonal therapy as fir...
2000-01-01 [J. Clin. Oncol. 18(2) , 262-6, (2000)] |
|
Toxic effects of anabolic-androgenic steroids in primary rat...
1995-08-01 [J. Pharmacol. Toxicol. Methods 33(4) , 187-95, (1995)] |
|
Randomized trial of tamoxifen alone or combined with fluoxym...
2006-07-01 [Breast Cancer Res. Treat. 98(2) , 217-22, (2006)] |