Predictors and correlates for weight changes in patients co-treated with olanzapine and weight mitigating agents; a post-hoc analysis.
Virginia L Stauffer, Ilya Lipkovich, Vicki Poole Hoffmann, Alexandra N Heinloth, H Scott McGregor, Bruce J Kinon
Index: BMC Psychiatry 9 , 12, (2009)
Full Text: HTML
Abstract
This study focuses on exploring the relationship between changes in appetite or eating behaviors and subsequent weight change for adult patients with schizophrenia or bipolar disorder treated with olanzapine and adjunctive potential weight mitigating pharmacotherapy. The aim is not to compare different weight mitigating agents, but to evaluate patients' characteristics and changes in their eating behaviors during treatment. Identification of patient subgroups with different degrees of susceptibility to the effect of weight mitigating agents during olanzapine treatment may aid clinicians in treatment decisions.Data were obtained from 3 randomized, double-blind, placebo-controlled, 16-week clinical trials. Included were 158 patients with schizophrenia or bipolar disorder and a body mass index (BMI) > or = 25 kg/m2 who had received olanzapine treatment in combination with nizatidine (n = 68), sibutramine (n = 42), or amantadine (n = 48). Individual patients were analyzed for categorical weight loss > or= 2 kg and weight gain > or = 1 kg. Variables that were evaluated as potential predictors of weight outcomes included baseline patient characteristics, factors of the Eating Inventory, individual items of the Eating Behavior Assessment, and the Visual Analog Scale.Predictors/correlates of weight loss > or = 2 kg included: high baseline BMI, low baseline interest in food, and a decrease from baseline to endpoint in appetite, hunger, or cravings for carbohydrates. Reduced cognitive restraint, increase in hunger, and increased overeating were associated with a higher probability of weight gain > or = 1 kg.The association between weight gain and lack of cognitive restraint in the presence of increased appetite suggests potential benefit of psychoeducational counseling in conjunction with adjunctive pharmacotherapeutic agents in limiting weight gain during antipsychotic drug therapy.This analysis was not a clinical trial and did not involve any medical intervention.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
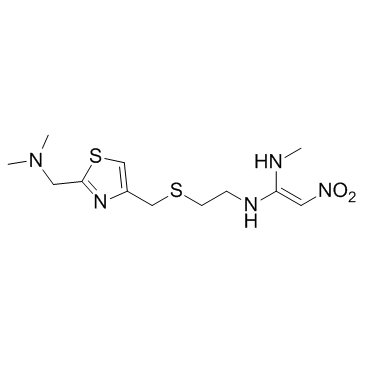 |
Nizatidine
CAS:76963-41-2 |
C12H21N5O2S2 |
|
Impact of sleep disorders in Japanese patients with function...
2013-08-01 [J. Gastroenterol. Hepatol. 28(8) , 1314-20, (2013)] |
|
[Nizatidine].
1989-01-01 [Medicina (Firenze.) 9(1) , 93-6, (1989)] |
|
Nizatidine improves clinical symptoms and gastric emptying i...
2012-01-01 [Digestion 86(2) , 114-21, (2012)] |
|
Onset of relief of symptoms of gastroesophageal reflux disea...
2010-04-01 [Clin. Ther. 32(4) , 678-90, (2010)] |
|
Weight gain management in patients with schizophrenia during...
2006-12-01 [Rev. Bras. Psiquiatr. 28(4) , 270-6, (2006)] |