Nizatidine
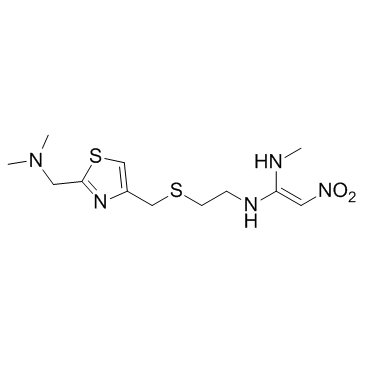
Nizatidine structure
|
Common Name | Nizatidine | ||
|---|---|---|---|---|
| CAS Number | 76963-41-2 | Molecular Weight | 331.457 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 478.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C12H21N5O2S2 | Melting Point | 130-132ºC | |
| MSDS | Chinese USA | Flash Point | 243.0±28.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Impact of sleep disorders in Japanese patients with functional dyspepsia (FD): nizatidine improves clinical symptoms, gastric emptying and sleep disorders in FD patients.
J. Gastroenterol. Hepatol. 28(8) , 1314-20, (2013) The association between functional dyspepsia (FD) and sleep disorders has yet to be studied in detail. The aim of this study is to evaluate the risk factors associated with sleep disorders and the clinical response to nizatidine therapy for sleep disorders in... |
|
|
[Nizatidine].
Medicina (Firenze.) 9(1) , 93-6, (1989) Nizatidine is a new H2-receptor antagonist, as potent as ranitidine. It does not interfere with hepatic metabolism and it lacks anti-androgenic effects. An evening dose of 300 mg suppresses nocturnal acid secretion without diurnal carryover. Published clinica... |
|
|
Nizatidine improves clinical symptoms and gastric emptying in patients with functional dyspepsia accompanied by impaired gastric emptying.
Digestion 86(2) , 114-21, (2012) In this crossover study, we investigated whether nizatidine, a H(2)-receptor antagonist, can alleviate clinical symptoms and gastric emptying in patients with Rome III-based functional dyspepsia (FD) with or without impaired gastric emptying.We enrolled 30 pa... |
|
|
Onset of relief of symptoms of gastroesophageal reflux disease: post hoc analysis of two previously published studies comparing pantoprazole 20 mg once daily with nizatidine or ranitidine 150 mg twice daily.
Clin. Ther. 32(4) , 678-90, (2010) Systematic assessments of the onset of symptom relief in the treatment of gastroesophageal reflux disease (GERD) are lacking.This work evaluated the time interval until complete symptom relief from heartburn (including both daytime and nighttime heartburn) an... |
|
|
Weight gain management in patients with schizophrenia during treatment with olanzapine in association with nizatidine.
Rev. Bras. Psiquiatr. 28(4) , 270-6, (2006) Weight gain is associated with treatment with many psychotropic agents. Nizatidine, H2 receptor antagonist, has been proposed to have weight-reducing effects. This was a 12-week, randomized, double-blind, placebo-controlled trial to evaluate the efficacy of n... |
|
|
Predictors and correlates for weight changes in patients co-treated with olanzapine and weight mitigating agents; a post-hoc analysis.
BMC Psychiatry 9 , 12, (2009) This study focuses on exploring the relationship between changes in appetite or eating behaviors and subsequent weight change for adult patients with schizophrenia or bipolar disorder treated with olanzapine and adjunctive potential weight mitigating pharmaco... |
|
|
[Safety of proton pump inhibitors].
Med. Clin. (Barc.) 127(20) , 790-5, (2006) The significant inhibitory capacity of gastric acid secretion of PPIs makes them the drugs of choice for treating acid-related diseases. The considerable prevalence of these diseases and the need for maintaining the administration of the drug during considera... |
|
|
Prokinetic activity of nizatidine: implications for the management of patients with gastroesophageal reflux disease.
Clin. Ther. 21(12) , 2038-46; discussion 2037, (1999) Gastroesophageal reflux is a common condition caused mainly by motility disorders of the upper gastrointestinal tract. The most effective therapy combines acid suppression with a promotility agent. Nizatidine is a well-tolerated and effective histamine-2 (H2)... |
|
|
Multicenter, double-blind, placebo-controlled crossover study to assess the acute prokinetic efficacy of nizatidine-controlled release (150 and 300 mg) in patients with gastroesophageal reflux disease.
Am. J. Med. Sci. 340(4) , 259-63, (2010) The aim of the study is to test whether nizatidine delivered via a unique bimodal pulsatile-controlled release system, nizatidine controlled release (CR), accelerates gastric emptying in patients with gastroesophageal reflux disease (GERD).Combined data were ... |
|
|
Validated stability-indicating methods for the determination of nizatidine in the presence of its sulfoxide derivative.
J. AOAC Int. 91(1) , 73-82, (2008) Four new selective, precise, and accurate methods are described for the determination of nizatidine (NIZ) in the presence of its sulfoxide derivative in both the raw material and pharmaceutical preparations. Method A is based on zero-order (0D), first-derivat... |