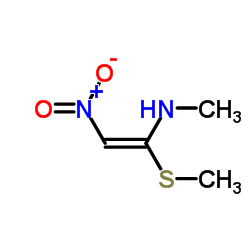
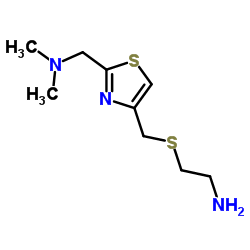
Nizatidine
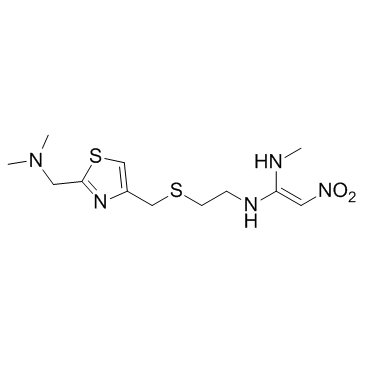
Nizatidine structure
|
Common Name | Nizatidine | ||
|---|---|---|---|---|
| CAS Number | 76963-41-2 | Molecular Weight | 331.457 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 478.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C12H21N5O2S2 | Melting Point | 130-132ºC | |
| MSDS | Chinese USA | Flash Point | 243.0±28.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of NizatidineNizatidine is a histamine H2 receptor antagonist with low toxicity that inhibits gastric acid secretion.Target: Histamine H2 ReceptorNizatidine, a selective histamine H2-receptor antagonist, is a potent inhibitor of gastric acid secretion, with IC50 of 0.9 nM. Nizatidine exhibits maximal inhibition of gastric acid in rats within the first hour of drug administration, with EC50 of 1.383 μmol/kg [1]. Nizatidine also reversibly inhibits acetylcholinesterase (AChE), with IC50 of 6.7 μM, and the inhibition is noncompetitive, with a Ki value of 7.4 μM. Nizatidine (0.3-3 mg/kg, i.v.) significantly increases the motor index of gastrointestinal (GI) motility in a dose-dependent manner. Nizatidine inhibits gastric acid secretion with ED50 and ED90 of 0.18 and 3.22 mg/kg in dogs, and 2.94 and 19.6 mg/kg in rats, respectively [2]. |
| Name | nizatidine |
|---|---|
| Synonym | More Synonyms |
| Description | Nizatidine is a histamine H2 receptor antagonist with low toxicity that inhibits gastric acid secretion.Target: Histamine H2 ReceptorNizatidine, a selective histamine H2-receptor antagonist, is a potent inhibitor of gastric acid secretion, with IC50 of 0.9 nM. Nizatidine exhibits maximal inhibition of gastric acid in rats within the first hour of drug administration, with EC50 of 1.383 μmol/kg [1]. Nizatidine also reversibly inhibits acetylcholinesterase (AChE), with IC50 of 6.7 μM, and the inhibition is noncompetitive, with a Ki value of 7.4 μM. Nizatidine (0.3-3 mg/kg, i.v.) significantly increases the motor index of gastrointestinal (GI) motility in a dose-dependent manner. Nizatidine inhibits gastric acid secretion with ED50 and ED90 of 0.18 and 3.22 mg/kg in dogs, and 2.94 and 19.6 mg/kg in rats, respectively [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 478.2±45.0 °C at 760 mmHg |
| Melting Point | 130-132ºC |
| Molecular Formula | C12H21N5O2S2 |
| Molecular Weight | 331.457 |
| Flash Point | 243.0±28.7 °C |
| Exact Mass | 331.113678 |
| PSA | 139.55000 |
| LogP | 1.18 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.592 |
| InChIKey | SGXXNSQHWDMGGP-IZZDOVSWSA-N |
| SMILES | CNC(=C[N+](=O)[O-])NCCSCc1csc(CN(C)C)n1 |
| Storage condition | -20?C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~78% 
Nizatidine CAS#:76963-41-2 |
| Literature: KUMAR, Dinesh, R. Patent: WO2004/69817 A1, 2004 ; Location in patent: Page 8,9 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Impact of sleep disorders in Japanese patients with functional dyspepsia (FD): nizatidine improves clinical symptoms, gastric emptying and sleep disorders in FD patients.
J. Gastroenterol. Hepatol. 28(8) , 1314-20, (2013) The association between functional dyspepsia (FD) and sleep disorders has yet to be studied in detail. The aim of this study is to evaluate the risk factors associated with sleep disorders and the cli... |
|
|
[Nizatidine].
Medicina (Firenze.) 9(1) , 93-6, (1989) Nizatidine is a new H2-receptor antagonist, as potent as ranitidine. It does not interfere with hepatic metabolism and it lacks anti-androgenic effects. An evening dose of 300 mg suppresses nocturnal ... |
|
|
Nizatidine improves clinical symptoms and gastric emptying in patients with functional dyspepsia accompanied by impaired gastric emptying.
Digestion 86(2) , 114-21, (2012) In this crossover study, we investigated whether nizatidine, a H(2)-receptor antagonist, can alleviate clinical symptoms and gastric emptying in patients with Rome III-based functional dyspepsia (FD) ... |
| Tazac |
| N-{2-[({2-[(Dimethylamino)methyl]-1,3-thiazol-4-yl}methyl)sulfanyl]ethyl}-N'-methyl-2-nitroethene-1,1-diamine |
| Acinon |
| (E)-1-N'-[2-[[2-[(dimethylamino)methyl]-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-1-N-methyl-2-nitroethene-1,1-diamine |
| Nizax |
| N-[2[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine |
| Cronizat |
| Axid |
| N-{2-[({2-[(Dimethylamino)methyl]-1,3-thiazol-4-yl}methyl)sulfanyl]ethyl}-N'-methyl-2-nitro-1,1-ethenediamine |
| MFCD00865660 |
| Galitidin |
| Distaxid |
| 1,1-Ethenediamine, N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N'-methyl-2-nitro- |
| Nizatidine |
| Zanizal |
| Calmaxid |
| 1,1-Ethenediamine, N-(2-(((2-((dimethylamino)methyl)-4-thiazolyl)methyl)thio)ethyl)-N'-methyl-2-nitro- |