Palladium-catalyzed cross-coupling of electron-poor terminal alkynes with arylboronic acids under ligand-free and aerobic conditions.
Ming-Bo Zhou, Wen-Ting Wei, Ye-Xiang Xie, Yong Lei, Jin-Heng Li
Index: J. Org. Chem. 76th ed., 75 , 5635-5642, (2010)
Full Text: HTML
Abstract
Palladium-catalyzed cross-coupling reaction of terminal alkynes with arylboronic acids has been described. In the presence of Pd(OAc)(2) and Ag(2)O, a variety of terminal alkynes, including electron-poor terminal alkynes, smoothly underwent the reaction with numerous boronic acids to afford the corresponding internal alkynes in moderate to good yields. Moreover, this methodology was applied to the synthesis of 1H-isochromenes and diynes. It is noteworthy that the reaction proceeds under ligand-free and relative lower loading Pd conditions, and the maximal TONs (turnover numbers) of the reaction are up to 720,000.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
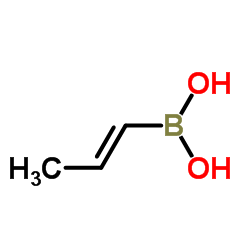 |
(1E)-1-Propen-1-ylboronic acid
CAS:7547-97-9 |
C3H7BO2 |
|
Discovery of potent, selective, and orally bioavailable alky...
2011-10-27 [J. Med. Chem. 20th ed., 54 , 7299-7317, (2011)] |
|
Selective palladium-catalyzed C-F activation/carbon-carbon b...
2012-02-17 [J. Org. Chem. 4th ed., 77 , 1798-1804, (2012)] |
|
Highly selective nickel-catalyzed three-component coupling o...
2010-08-20 [Org. Lett. 12 , 3610-3613, (2010)] |
|
The preparation of substituted pyrazoles from β,β-...
[Synlett 4 , 602-606, (2010)] |
|
A new strategy for the synthesis of substituted dihydropyron...
[Tetrahedron 67 , 4995-5010, (2011)] |