[Aspartyladenylate analog--effective inhibitor of asparagine synthetase].
Iu N Zhukov, A I Biriukov, R M Khomutov
Index: Bioorg. Khim. 14(7) , 969-72, (1988)
Full Text: HTML
Abstract
A number of earlier unknown phosphonate analogues of aspartyl adenylate with anhydride oxygen substituted by --CH2--, and the carbonyl group substituted by --CH(OH)- or --CH(NH2)-groups were synthesized. These compounds were used to study the reaction mechanism of asparagine synthetases from white lupine and E. coli. The aspartyl adenylate analogues proved to be powerful competitive inhibitors (Ki = 10(-7) M) of the bacterial enzyme. In the case of white lupine enzyme catalyzing the aspartate-independent ATP--[32P]PPi exchange, the above compounds displayed a non-competitive type of inhibition with respect to aspartate and ATP, Ki = 10(-4) M. It is likely that for the latter enzyme the first intermediate is different from an aspartyl adenylate derivative.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
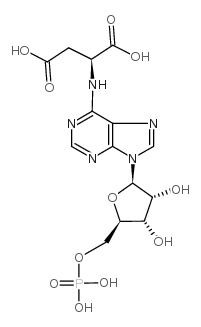 |
Adenylosuccinic acid
CAS:19046-78-7 |
C14H18N5O11P |
|
The RimL transacetylase provides resistance to translation i...
2014-10-01 [J. Bacteriol. 196(19) , 3377-85, (2014)] |
|
Synthesis and aminoacyl-tRNA synthetase inhibitory activity ...
2005-01-03 [Bioorg. Med. Chem. 13(1) , 69-75, (2005)] |
|
MccE provides resistance to protein synthesis inhibitor micr...
2010-04-23 [J. Biol. Chem. 285(17) , 12662-9, (2010)] |
|
Escherichia coli peptidase A, B, or N can process translatio...
2008-04-01 [J. Bacteriol. 190(7) , 2607-10, (2008)] |
|
Aspartyl tRNA-synthetase from Escherichia coli: flexibility ...
2000-06-23 [J. Mol. Biol. 299(5) , 1157-64, (2000)] |